Changes in Levels of Gene Expression in Human Aortal Intima during Atherogenesis
T. A. Shchelkunova1, I. A. Morozov2, P. M. Rubtsov2, L. M. Samokhodskaya3, I. V. Andrianova4, I. A. Sobenin5, A. N. Orekhov5, and A. N. Smirnov1*
1Biological Faculty, Lomonosov Moscow State University, Leninsky Gori 1/12, 119899 Moscow, Russia; fax: (495) 939-4309; E-mail: smirnov__an@mail.ru2Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, ul. Vavilova 32, 119899 Moscow, Russia; fax: (499) 135-1405; E-mail: rubtsov@eimb.ru
3Faculty of Fundamental Medicine, Lomonosov Moscow State University, Lomonosovsky pr. 31/5, 119899 Moscow, Russia; fax: (495) 932-9904; E-mail: slm@fbm.msu.ru
4Russian Cardiological Scientific Production Complex, 3-ya Cherepkovskaya ul. 15A, 121552 Moscow, Russia; fax: (495) 414-6731; E-mail: irandrianova@yandex.ru
5Institute of General Pathology and Pathophysiology, Russian Academy of Medical Sciences, Baltiiskaya ul. 8, 125315 Moscow, Russia; fax: (495) 415-9594; E-mail: office@inat.ru
* To whom correspondence should be addressed.
Received December 17, 2012; Revision received January 21, 2013
Changes in the contents of 36 mRNAs species related to lipid turnover, inflammation, metabolism and the action of sex hormones in samples of aortal intima along the “intact tissue – lesions of type I – lesions of type II – lesions of type Va” sequence were analyzed using quantitative PCR. The expression of several mRNAs coding for components of the vesicular transfer and lipid turnover machinery was found to be resistant to atherogenesis or even decline in the course of atherogenesis. Decrease in expression was also recorded for steroid sulfatase, androgen receptor, and low density lipoprotein receptor mRNAs. However, the contents of the majority of other mRNA species increased gradually during disease progression. The earliest changes found as early as in lesions of type I were characteristic for estrogen sulfotransferase, apolipoprotein E, scavenger receptor SR-BI, collagen COL1A2, as well as chemokine CCL18 mRNAs. The contents of several mRNAs in intact tissue and atherosclerotic injuries had gender differences. Additionally, responses of two mRNAs, for aromatase and sterol regulatory element binding protein 2, to atherosclerotic lesion were also sex-differentiated. The contents of the majority of analyzed mRNAs in peripheral blood monocyte-derived macrophages were higher than in intact aorta. The correlations found in atherosclerotic lesions between mRNA species that predominant in macrophages and those expressed at comparable levels in macrophages and intact aorta or mainly in aorta suggest that the observed rise in the content of the majority of mRNAs during atherogenesis is determined by increase in expression in resident cells. The data suggest that the revealed absence of homeostatic regulation of expression of a number of genes associated with vesicular transfer and lipid turnover can serve as one of the reasons for lysosomal function insufficiency that leads to foam cell formation in atheroma. The observed sex differences in expression of a number of mRNAs suggest that estrogens in women perform their atheroprotective effects starting with predisposition to the disease and finishing with advanced stages of the pathologic process.
KEY WORDS: mRNA, PCR, gene expression, atherogenesis, aorta, gender differences, macrophagesDOI: 10.1134/S0006297913050040
Abbreviations (in brackets names of gene are given according to nomenclature of the database of University of California Santa Cruz (UCSC) Genome Browser (http://genome.ucsc.edu/)): ABCA1 and ABCG1, ATP-binding cassette transporters A1 and G1 (ABCA1, ABCG1); ACAT1, acyl-CoA-cholesterol acyltransferase 1 (ACAT1); ApoE, apolipoprotein E (APOE); AR, androgen receptor (AR); Arom, aromatase (CYP19A1); CCL18, C-C motif-containing chemokine 18 (CCL18); CD36, CD63, and CD68, differentiation clusters 36, 63, and 68 (CD36, CD63, CD68); CEH, cholesteryl ester neutral hydrolase (LIPA); COL1A2, α-2 type I collagen (COL1A2); EEA1, early endosome antigen 1 (EEA1); ERα, estrogen receptor alpha (ESR1); EST, estrogen sulfotransferase (SULT1E1); GAPDH, glyceraldehyde-3-phosphate dehydrogenase (GAPDH); GNB2L1, guanine nucleotide-binding protein, beta-peptide 2-like 1 (GNB2L1); ICAM1, intercellular adhesion molecule 1 (ICAM1); Lamp 1/2, lysosome-associated membrane glycoproteins 1 and 2 (LAMP1/2); LDLR, low density lipoprotein receptor (LDLR); LPL, lipoprotein lipase (LPL); LXRα and LXRβ, liver X receptors alpha and beta (NR1H3, NR1H2); p62, ubiquitin-binding protein p62 (SQSTM1); PPARα and PPARγ, peroxisome proliferators-activated receptors alpha and gamma (PPARA, PPARG); Rab5a, Ras-related small GTPase 5A (RAB5A); SELE, selectin E (SELE); SR-A, scavenger receptor A (MSR1); SR-BI, scavenger receptor BI (SCARB1); SREBP1 and SREBP2, sterol regulatory element-binding proteins 1 and 2 (SREBF1, SREBF2); STS, steroid sulfatase (STS); TfR1, transferrin receptor 1 (TFRC); TLR4, Toll-like receptor 4 (TLR4); TNFα, tumor necrosis factor alpha (TNF); VCAM1, vascular cell adhesion molecule 1 (VCAM1); mLDL, modified low density lipoproteins.
Atherosclerosis is one of the most frequent causes of death and
disablement. The disease affects large and middle-sized arterial
vessels usually in atherogenesis-prone regions that include
bifurcations and sharp turns of vessels. Blood turbulence is believed
to result in injury or change in characteristics of endothelium that is
a prerequisite for switching on factors of atherogenesis [1]. These factors comprise first of all disorders in
lipid metabolism and inflammation and oxidative stress inducers [2-4]. According to the American
Heart Association classification [5, 6], six (I-VI) morphological types of atherosclerotic
lesions are distinguished, types I, II, III, IV, and Va being
considered as corresponded to successive stages of the disorder. In
this sequence of lesions, progressive rise in lipid accumulation in
cells (foam cells) and extracellular lipid deposits as well as in
invasion of hematogenous cells including monocytes/macrophages
associated with local inflammation is observed. Lipid accumulation is
accompanied by increase in production of proinflammatory factors that
in turn stimulate further uptake of lipids, thus forming a vicious
circle. The concepts of functional ties between lipid metabolism and
inflammatory process are based mainly on data for macrophages that are
used as a surrogate model of atherogenesis [7, 8]. However, since in aortal atherosclerotic lesions
hematogenous cells represent only a small fraction of the total cell
population even in lesions of type Va [9], there is
need for knowledge of changes in metabolic processes in intimal
resident cells in the course of atherogenesis for fuller understanding
of the mechanisms of the disease.
The objective of this study was to analyze the contents of mRNAs coding for protein components of executive systems for cellular lipid uptake, intracellular lipid transfer and turnover, and reverse cholesterol transfer along with regulators of these systems represented by sensors of lipids in comparison with mRNAs coding for cell adhesion molecules and known proinflammatory factors. Taking into account gender differences in risks of atherosclerosis [10], analysis of the contents of mRNAs for receptors and enzymes of metabolism of sex hormones in intimal tissue was also performed in the study.
MATERIALS AND METHODS
Artery samples. This study used aorta samples extracted during autopsies from 48 (37 male and 11 female) donors aged 17 to 57 years 4-6 h after accidental death. These samples included 48 fragments of aorta without lesions, 21 fragments with lesions of type I, 29 fragments with lesions of type II, and 15 fragments with lesions of type Va. When comparisons between samples with different types of lesions were performed, pairs of fragments taken from the same donors were used. Twenty-one norm/lesion pairs of type I, 29 norm/lesion pairs of type II, and 15 norm/lesion pairs of type Va were analyzed. The vessels were washed with PBS and dissected longitudinally for biochemical and histological analysis. For mRNA measurements, the intima from apparently normal and atherosclerotically damaged areas were mechanically separated, frozen in liquid nitrogen, and kept at –70ºC before analysis. No lesion shoulders were taken for analysis. The ascribing of samples to certain morphological types of atherosclerotic damages was confirmed microscopically according to the American Heart Association classification [5, 6].
Isolation of monocyte-derived macrophages. Mononuclear cells were isolated from fasting venous blood of 17 healthy volunteers (9 women and 8 men) aged 21-57 years with their written informed consent. The isolation was performed by centrifugation on a Ficoll-Paque Plus (Amersham Biosciences, Sweden) density gradient according the manufacturer’s protocol.
To prepare monocyte-derived macrophages, mononuclear cell suspensions in DMEM (Gibco BRL Life Technologies, USA) containing 10% fetal bovine serum (ICN Biochemicals Inc., USA), 2 mM glutamine, 100 mg/ml kanamycin, and 2.5 mg/ml fungizone were plated onto 35-mm plastic culture dishes (Corning Costar, USA) at a density of 3·106 cells per dish. Cells were cultivated at 100% humidity and 37ºC in a CO2 incubator (5% CO2 and 95% atmospheric air) for 7-10 days with medium changes every second day.
Macrophage loading with lipids. Lipid-loaded monocyte-derived macrophages were prepared using freshly isolated naturally modified low density lipoproteins (mLDLs), which were isolated from pooled serum of patients with diagnosed atherosclerosis (with their written informed consent) by two-stage ultracentrifugation in a discontinuous density gradient of sodium bromide. The LDL fraction thus obtained was dialyzed against PBS for 12 h at 4ºC and sterilized by filtration using a pore size of 0.45 μm. mLDLs at final concentration 100 μg/ml were added to the cells and incubated for 18 h. Cells were then washed three times with PBS, lysed with Trizol, and kept at –70ºC. An aliquot of the cell preparation was used for cholesterol measurements.
To confirm mLDL-induced cholesterol accumulation in macrophages, cells were extracted three times with hexane–isopropanol mixture (3 : 2 v/v). The cholesterol content was determined enzymatically using a reagent kit from Biocon Diagnostik (Germany) and normalized to the protein content in each sample measured by the Lowry method [11].
mRNA measurements. mRNA contents were measured using quantitative real-time polymerase chain reaction. The details of analysis were described earlier [12]. Briefly, RNA was isolated from frozen samples using TRIzol Reagent (Invitrogen, USA). cDNA was synthesized on total RNA using a Promega ImProm_II Reverse Transcription System kit (Promega, USA). The synthesized cDNA was used as a template for real-time PCR (qRT-PCR) on a Rotor-Gene 3000 amplifier (Corbett Research, Australia) with a kit of reagents including the intercalating dye SYBR Green I (Syntol, Russia) as recommended by the manufacturer. Used primers were those described by us previously [13] as well as published primers for guanine nucleotide-binding protein, beta-peptide 2-like 1 (GNB2L1) [14] and α-2 type I collagen (COL1A2) [15]. Amplified products were sequenced using an ABI PRISM BigDye Terminator v.3.1 kit (Applied Biosystems, USA) of reagents and an ABI PRISM 3100-Avant automated DNA sequencer (Applied Biosystems) to confirm the expected sequence. The results of PCR were included only when the melting temperature and the electrophoretic mobility of the amplified products corresponded to the expected values. The contents of individual mRNAs were expressed as percent of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA content used as an internal reference control.
Statistics. Less than half of the data for mRNA contents in samples corresponded to normal distribution. Therefore, correlations between values were evaluated using the Spearman rank correlation coefficient (Rs), and differences in the contents of mRNAs were evaluated using the Mann–Whitney U-test. For analysis, data for atherosclerotic lesion/intact tissue pairs taken from the same donor were used. The results were analyzed with the Statistica 7.0 program. Differences or correlations were considered significant at p < 0.05.
RESULTS
GAPDH mRNA as common reference. GAPDH mRNA is often used as an internal reference control for normalizing the contents of other mRNA species in different tissues. To validate the relevance of GAPDH mRNA as common reference point for intact and atherosclerotically injured aortal tissue as well as macrophages, two approaches were used.
First, we analyzed the expression of the additional housekeeping gene, GNB2L1, in pairs of aortal fragments from several donors. The ratios of GNB2L1 mRNA contents in injuries to its contents in intact aorta fragments, as normalized by GAPDH mRNA, were close to 1.0 for lesions of type I, II, and Va (0.94 ± 0.38 (n = 6), 0.96 ± 0.24 (n = 6), and 0.88 ± 0.11 (n = 4), respectively). The data suggest that the expression of the two housekeeping genes does not vary significantly during atherogenesis.
Second, an experiment with mixing of cDNAs from intact aorta and lipid-loaded macrophages at 9 : 1 was performed. The contents of amplified products for 15 mRNA species in this mix normalized by GAPDH mRNA corresponded to the levels expected on the basis of results for separate measurements of analyzed mRNAs in macrophages and aorta (observed/expected ratio value was 0.99 ± 0.19 (mean ± SD)). The results suggest that GAPDH mRNA is expressed at similar levels in macrophages and aortal tissue.
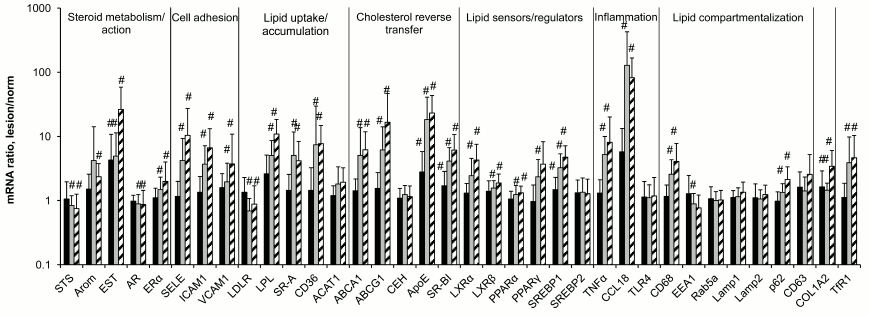
Evolution in contents of mRNAs during atherogenesis. The data of Fig. 1 on the contents of 36 mRNA species in aortal intima show that expression of the majority of these mRNAs changes during atherogenesis. No significant changes were recorded for mRNAs coding for enzymes of cholesterol esterification and hydrolysis of these esters (ACAT1 and CEH, respectively), components of the system of vesicular transfer and lipid processing (Rab5a, Lamp1, Lamp2, CD63), transcriptional factor SREBP2 that takes a part in regulation of lipid metabolism, and sensor of bacterial lipopolysaccharides TLR4. Lowering in expression was observed for mRNAs coding for steroid sulfatase (STS), androgen receptor (AR), low density lipoprotein receptor (LDLR), and early endosome antigen 1 (EEA1). The contents of 24 other studied mRNA species increased during atherogenesis.
Fig. 1. Ratios of mRNAs contents in atherosclerotic lesion–intact aorta sample pairs taken from the same donors. Black bars, lesion of type I; gray bars, lesion of type II; hatched bars, lesion of type Va. The content of each mRNA in intact aorta was accepted as 1.0. Symbol # marks values significantly different from 1.0.
The most sensitive to atherosclerotic lesion were mRNAs coding for the enzyme of estrogen conjugation (EST), the components of reverse cholesterol transfer ApoE and SR-BI, as well as the main protein component of extracellular matrix in vessel collagen 1A2: the rise in their contents were found as early as in lesions of type I. Chemokine CCL18 mRNA joins to this group; its content was obviously although not significantly elevated in lesions of type I. Significant changes in the contents of the majority of other mRNA species were observed only in lesions of type II and Va. In terms of extent of changes in the course of atherogenesis (exceeding one order in magnitude), mentioned above EST, ApoE, and CCL18 as well as selectin E (SELE) and lipoprotein lipase (LPL) mRNAs were leaders, CCL18 mRNA being the absolute leader with approximately 100-fold rise in lesions of types II and Va over intact aortal tissue, which corresponds with the data of others [16].
Age and gender features of mRNA expression in aorta. The above results relate to combined data for all samples of aorta studied including samples from men and women of different ages.
Analysis showed that the contents of two mRNA species in intact aorta weakly but significantly correlated with age: STS Rs = 0.33; SELE Rs = –0.39. These correlations were significant in Pearson regression analysis as well (both sets of values corresponded to normal distribution).
The contents of several mRNAs were sex differentiated in samples without lesions and harboring atherosclerotic lesions (Table 1).
Table 1. Gender differences in contents of
mRNAs found in aorta samples
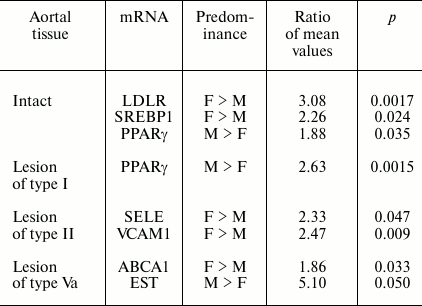
In addition, for two mRNAs gender differences in ratios of their contents in lesions of type II and intact aortal tissue were revealed (Table 2). Since the number of lesion/norm sample pairs from women (8) was small compared to the number of such pairs from men (20), in combined sampling men + women no significant differences of ratios of type II lesion/norm from 1.0 for Arom and SREBP2 mRNAs were found (see Fig. 1).
Table 2. Gender differences in ratios of
mRNA contents found in lesion and intact aorta tissue
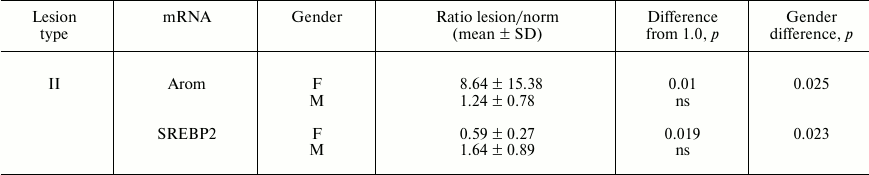
Note: ns, not significant.
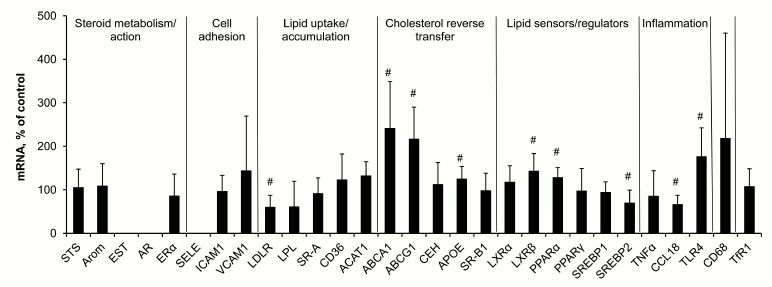
Macrophage response to lipid loading. Incubation of macrophages with modified low density lipoproteins for 18 h resulted in 2- to 3-fold rise in the content of cholesterol in cells and was accompanied by changes in expression of several mRNA species (Fig. 2), the contents of LDLR, SREBP2, and CCL18 mRNAs decreasing while the levels of mRNAs for ATP-binding cholesterol transporters ABCA1 and ABCG1, ApoE, oxysterol sensor LXRβ, fatty acid sensor PPARα, and Toll-like receptor 4 (TLR4) increasing.
Fig. 2. Changes in contents of mRNAs in macrophages induced by cell incubation with modified low density lipoproteins. Symbol # marks values significantly different from 100%.
Comparison of these lipid-induced shifts in mRNA expression in macrophages and observed differences between lesions of types II and Va and intact tissue (Fig. 1) showed the presence of some shared features in regulation of gene expression. Decrease in the content of LDLR mRNA and increase in the levels of mRNAs for ABCA1, ABCG1, ApoE, LXRβ, and PPARα found in both cases seem to be a part of homeostatic response to rise in cellular lipids. However, the response of macrophages to lipid challenge differed from that of aortal cells in terms of expression of mRNAs coding inflammatory reaction components, CCL18 and TLR4. CCL18 mRNA was the leader in the extent of rise in atherosclerotic lesions compared to intact aorta, while in macrophages CCL18 mRNA expression decreased after lipid loading. And on the contrary, the level of TLR4 mRNA in macrophages after lipid challenge increased, while no change in its content in aorta under atherogenesis was recorded.
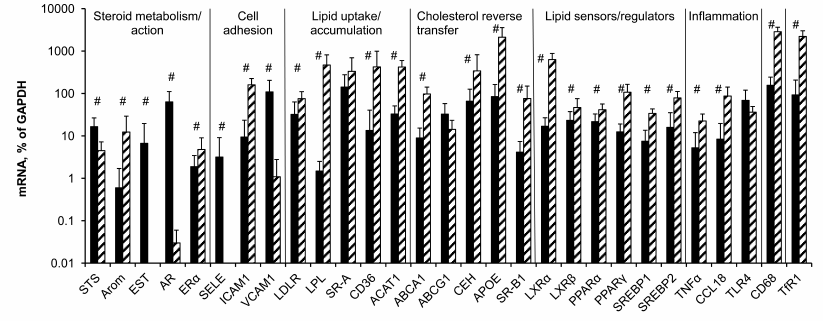
Potential contribution of macrophages in measured levels of mRNAs in aorta. The data of Fig. 3 show that with few exceptions mRNA levels in control macrophages and intact aorta differ significantly. No differences were found only for SR-A, ABCG1, and TLR4 mRNAs. Unlike aorta, in macrophages the expression of EST, AR, and SELE mRNAs was extremely low. Compared to macrophages, in aorta STS and VCAM1 mRNAs were expressed at higher levels as well. However, the majority of the studied mRNAs expressed in macrophages at higher levels compared to aortal tissue.
Fig. 3. Contents of mRNAs in intact aorta (black bars) and control macrophages (hatched bars). Symbol # marks significant differences between contents of mRNAs in aorta and macrophages.
When the difference in the content of mRNA is one order of magnitude or more (Arom, ICAM1, LPL, CD36, ACAT, ApoE, SR-BI, LXRα, PPARγ, CCL18, CD68, TfR1), there is a probability that the observed changes in the content of mRNA during atherogenesis partially result from bringing in these mRNAs by monocytes/macrophages, which enter atherosclerotic lesions from the bloodstream. Among approaches to remove the problem of macrophage contribution to evolution of mRNA expression in atherosclerotic lesions is analysis of the presence or absence of correlations for mRNA pairs, where the first mRNA is expressed in macrophages at low level if at all (EST, AR, SELE, VCAM1, STS) or whose expression does not differ from that in aorta (SR-A, ABCG1, TLR4) and the second mRNA obviously predominates in macrophages. Such correlations could arise when both mRNAs of a pair were expressed in the same cells.
As shown in Table 3, in atherosclerotic lesions all mRNA species that were expressed predominantly in macrophages had correlations with mRNAs whose expression was more specific for aorta or equal to expression in macrophages. These results suggest that the rise in mRNA contents in atherosclerotic lesions compared to intact aorta does not relate to bringing in these mRNAs by macrophages. It is also obvious that the rise in the contents of aorta tissue-specific EST, SELE, and VCAM1 mRNAs in atherosclerotic lesions especially is not associated with macrophagic invasion.
Table 3. Correlations in atherosclerotic
lesions between contents of mRNAs expressed predominantly in aortal
tissue (Aor) or macrophages (MPh)
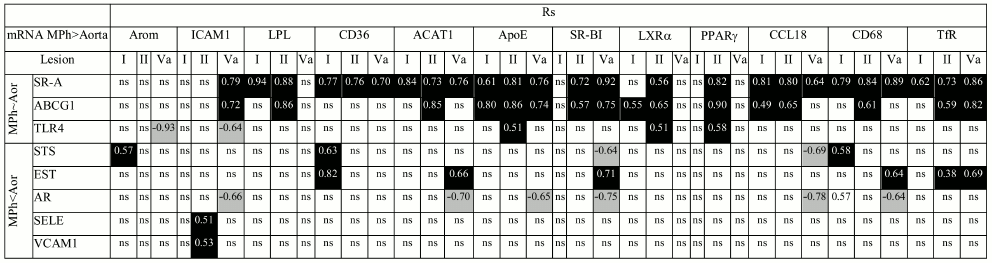
Note: Positive and negative correlations are shown in black and grey
boxes, respectively; ns, not significant.
DISCUSSION
The absence of changes in contents of mRNAs coding a number of components of endosomes and lysosomes (Rab5a, Lamp1, Lamp2, CD63) during atherogenesis is an interesting result of the study. Moreover, the content of mRNA for early endosome component EEA1 in atherosclerotic lesions was found to even be lowered (Fig. 1). Thus, the expression of genes associated with vesicular transfer of lipids coming into the cell and their following processing in lysosomes in atherosclerotic lesions does not correspond to the expected homeostatic regulation in response to enhanced lipid entrance into the cell. Homeostatic regulation was also not found for the contents of ACAT1 and CEH mRNAs, two enzymes that relate to lipid turnover in lipid droplets. We do not know if the levels or activities of these proteins always correspond to the contents of their mRNAs. If so, the absence of homeostatic regulation for these mRNAs could serve as a cause of low efficiency of pronounced homeostatic responses of mRNAs coding executive components of reverse cholesterol transfer (ABCA1, ABCG1, ApoE, SR-BI), lipid uptake by cells (LDLR), and their transcriptional regulators including lipid sensors (LXRα, LXRβ, PPARα, PPARγ, SREBP1). The assumption of lysosomal function insufficiency as a cause of foam cell formation in atherogenesis was proposed long ago [17, 18], but the specific mechanism of this insufficiency is still unclear. The results of the present study suggest that the absence of adaptive biogenesis of lysosomes under circumstances of elevated lipid entrance into cells can serve as such a mechanism.
The other aspect of the results that needs special discussion is the revealed gender difference in the contents of mRNAs in intact aortal tissue and in atherosclerotic lesions as well as the data on the evolution of the contents of mRNAs coding receptors and enzymes of metabolism of sex hormones in aorta during atherogenesis. Atherosclerosis belongs to a group of diseases with marked gender differences in frequency of their development [19, 20]. Data of epidemiological studies point to estrogens as the main contributors to these differences: the lowering in their levels in menopause in women is accompanied by rapid rise in risk of development of the disease [21]. Many risk factors including levels and ratios of high and low density lipoproteins and blood pressure are targets for estrogens [22]. Protective effect of estrogens is believed to be a sum of their direct action on vessels and effects outside vessels, first of all in the liver [23]. One such effect is the stimulation of expression in hepatocytes of low density lipoprotein receptor (LDLR) that provides elimination of dangerous for vessels cholesterol from the blood. The mechanism of induction includes the interaction of estrogen receptor with the promoter region of the human LDLR gene containing neighboring binding sites for positive regulators SREBP1 and Sp1 [24, 25]. Thereupon, the revealed here predominance of LDLR and SREBP1 mRNAs in samples of intact aortas from women over their contents in aortas from men (Table 1) looks rather interesting. It is very likely that the mechanism of formation of gender difference in LDLR mRNA levels is similar to the mechanism found in hepatocytes. However, a question on possible role of sex differentiation in expression of LDLR (and SREBP1) in aorta for atherogenesis seems to be more important. It is believed that low density lipoproteins (LDL) become atherogenic only after modifications (oxidation, acetylation, glycation, desialation, proteolysis, aggregation, etc.), which take place extracellularly via enzymatic and non-enzymatic reactions [26-28]. These modifications are facilitated by the retention of LDL in sub-endothelial space due to interaction with components of extracellular matrix [30]. One can speculate that higher expression of LDLR in aortal intima in women compared to men can promote quicker uptake of LDL by cells, thus hindering atherogenic modifications of LDL.
In already formed atherosclerotic lesions, sex differences in the contents of several mRNA species are observed as well (Table 1). The most significant difference in magnitude concerns about 5-fold predominance in the content of EST mRNA in lesions of type Va from men over its level in samples from women. The tendency to this sex difference was also noted in intact aorta and lesions of types I and II (the ratios of mean values in men and women are 2.6, 6.2, and 3.5, respectively) although this difference was not statistically significant. EST catalyzes the estrogen inactivation reaction; therefore, the observed difference should promote redoubling in gender differences in estrogen action on vessel wall. The results suggest that sex hormones can control not only predisposition to atherosclerosis, but also development of already formed atherosclerotic lesion. The data on the presence of differences in the contents of Arom and SREBP2 mRNAs between lesions of type II and intact aorta only in samples from women (Table 2) can serve as a confirmation of this assumption. An enhancement in expression of Arom, which catalyzes production of estrogens from androgens, can compensate to some extent the rise in its inactivation by EST. Together with the above-discussed data on sex difference in EST expression, these results may indicate certain advantages of women over men in terms of preservation of susceptibility of atherosclerotic lesions to beneficial effects of estrogens. The reduced content of SREBP2 mRNA in samples of lesion of type II versus intact aorta taken from women but not from men can be contemplated as one such benefit. The targets for stimulatory effects of activated (nuclear) form of SREBP2 are mainly genes coding for enzymes of cholesterol biosynthesis [31], and so the decrease in SREBP2 mRNA can be an element of homeostatic regulation in response to enhanced entrance of cholesterol into cells of atherosclerotic lesion. Such homeostatic regulation has been demonstrated in HeLa cells [32] and macrophages (Fig. 2). The lowered expression of SREBP2 in lesions of type II from women can render them one more benefit: the lowering of expression of micro-RNA (miR-33) coded by intron 16 of the SREBP2 gene, which inhibits reverse cholesterol transfer by interaction with mRNA for transporter ABCA1 [33].
Cellular composition of arterial intima undergoes complex changes during atherogenesis due to infiltration of hematogenic cells (mainly monocytes/macrophages), migration of smooth muscle cells from media, proliferation, and change in cell phenotype [34]. Thus, smooth muscle cells can acquire certain features of macrophages, specifically the ability for phagocytosis and expression of macrophage marker CD68 [35]. Therefore, an interpretation of results on evolution in mRNA expression during atherogenesis is a rather difficult task. In the case of aorta, this task seems somehow simpler when compared to coronary and carotid arteries since even in lesions of type Va, hematogenic cells do not exceed 15% of the total cell population [9], while in coronary and carotid arteries their portion is 2- and 3-fold higher, respectively [34]. The comparison between the contents of a number of mRNAs in aortal intima and macrophages showed that the majority of these mRNAs is expressed in macrophages at higher levels (Fig. 3). To resolve the question of whether found changes in mRNA contents in intima during atherogenesis result from their bringing in by infiltrated macrophages, we analyzed correlations between the contents of mRNA species specific mainly for macrophages and mRNAs whose expression predominates in intact aortal intima or is similar in macrophages and aorta. The revealing of such correlations (Table 3) suggested that the observed changes in mRNA levels during atherogenesis are associated mainly with resident cells of the aortal intima. This conclusion is not doubtless, however. First, such correlations can arise not only due to the action of intracellular regulators that uniformly affect specific groups of genes, but also as a result of interactions between cells of different types, for example, via effects in resident cells induced by cytokines produced by macrophages. Second, the values of mRNA contents in macrophages have only approximate character, since macrophages under the influence of various factors can produce different phenotypes, M1 and M2 being the best known. In atherosclerotic lesions, simultaneous presence of macrophages of different phenotypes and, consequently, with different levels of gene expression has been documented [36, 37]. Therefore, ascribing of SR-A, ABCG1, and TLR4 mRNAs to a group with similar expression in macrophages and intimal resident cells is rather conditional. Nevertheless, the data on correlations between the contents of a number of mRNAs (Arom, ICAM1, TfR1) that evidently predominate in macrophages found in intact aorta and atherosclerotic lesions (data not shown) can be interpreted as a confirmation of the assumption that changes in the levels of mRNAs observed during atherogenesis are associated predominantly with resident cells of the aortal intima.
This work was financially supported by the Russian Foundation for Basic Research (project 09-04-00329-a).
Some of the experiments were performed using facilities of the Center of Collective Use “Genome” at the Engelhardt Institute of Molecular Biology.
REFERENCES
1.Libby, P. (2000) J. Intern. Med.,
247, 349-358.
2.Steinberg, D. (2009) J. Lipid Res.,
50, S376-381.
3.Van der Valk, F. M., van Wijk, D. F., and Stroes,
E. S. (2012) Curr. Opin. Lipidol., 23, 532-539.
4.Riccioni, G., and Sblendorio, V. (2012) J.
Geriatr. Cardiol., 9, 305-317.
5.Stary, H. C., Chandler, A. B., Glagov, S., Guyton,
J. R., Insull, W., Jr., Rosenfeld, M. E., Schaffer, S. A., Schwartz, C.
J., Wagner, W. D., and Wissler, R. W. (1994) Arterioscler.
Thromb., 14, 840-856.
6.Stary, H. C., Chandler, A. B., Dinsmore, R. E.,
Fuster, V., Glagov, S., Insull, W., Jr., Rosenfeld, M. E., Schwartz, C.
J., Wagner, W. D., and Wissler, R. W. (1995) Arterioscler. Thromb.
Vasc. Biol., 15, 1512-1531.
7.Smirnov, A. N. (2010) Biochemistry (Moscow),
75, 793-810.
8.Feig, J. E., and Feig, J. L. (2012) Front.
Physiol., 3, 286.
9.Andreeva, E. R., Mikhailova, I. A., Pugach, I. M.,
and Orekhov, A. N. (1999) Angiol. Vasc. Surg., 5
(Suppl.), 6-26.
10.Villablanca, A. C., Jayachandran, M., and Banka,
C. (2010) Clin. Sci. (London), 119, 493-513.
11.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) J. Biol. Chem., 193, 265-275.
12.Shchelkunova, T. A., Morozov, I. A., Rubtsov, P.
M., Samokhodskaya, L. M., Kireev, R. A., Andrianova, I. V., Orekhov, A.
N., and Smirnov, A. N. (2008) Biochemistry (Moscow), 73,
920-928.
13.Shchelkunova, T. A., Albert, E. A., Morozov, I.
A., Rubtsov, P. M., Samokhodskaya, L. M., Sobenin, I. A., Orekhov,
A. N., and Smirnov, A. N. (2011) Biochemistry (Moscow),
76, 1178-1184.
14.Ishii, T., Wallace, A. M., Zhang, X., Gosselink,
J., Abboud, R. T., English, J. C., Pare, P. D., and Sandford, A. J.
(2006) Eur. Respir. J., 27, 300-306.
15.Luzina, I. G., Tsymbalyuk, N., Choi, J., Hasday,
J. D., and Atamas, S. P. J. (2006) Cell. Physiol., 206,
221-228.
16.Hagg, D. A., Olson, F. J., Kjelldahl, J.,
Jernas, M., Thelle, D. S., Carlsson, L. M., Fagerberg, B.,
and Svensson, P. A. (2009) Atherosclerosis, 204,
e15-20.
17.De Duve, C. (1966) Proc. Inst. Med. Chic.,
26, 73-76.
18.De Duve, C. (1974) Acta Cardiol. Suppl.,
20, 9-25.
19.Banos, G., Guarner, V., and Perez-Torres, I.
(2011) Cardiovasc. Hematol. Agents Med. Chem., 9,
137-146.
20.Virdis, A., and Taddei, S. (2012)
Maturitas, 71, 326-330.
21.Vitale, C., Mendelsohn, M. E., and Rosano,
G. M. (2009) Nat. Rev. Cardiol., 6, 532-542.
22.Mumford, S. L., Dasharathy, S., Pollack, A. Z.,
and Schisterman, E. F. (2011) Clin. Lipidol., 6,
225-234.
23.Rubanyi, G. M., Johns, A., and Kauser, K.
(2002) Vasc. Pharmacol., 38, 89-98.
24.Croston, G. E., Milan, L. B., Marschke, K. B.,
Reichman, M., and Briggs, M. R. (1997) Endocrinology,
138, 3779-3786.
25.Li, C., Briggs, M. R., Ahlborn, T. E., Kraemer,
F. B., and Liu, J. (2001) Endocrinology, 142,
1546-1553.
26.Tertov, V. V., Sobenin, I. A., and Orekhov,
A. N. (1992) Int. J. Tissue React., 14, 155-162.
27.Mertens, A., and Holvoet, P. (2001) FASEB
J., 15, 2073-2084.
28.Soran, H., and Durrington, P. N. (2011) Curr.
Opin. Lipidol., 22, 254-261.
29.Camejo, G., Olsson, U., Hurt-Camejo, E.,
Baharamian, N., and Bondjers, G. (2002) Atheroscler. Suppl.,
3, 3-9.
30.Sobenin, I. A., Suprun, I. V., Karagodin, V.
P., Feoktistov, A. S., Melnichenko, A. A., and Orekhov, A. N. J. (2011)
Lipids, 2011, 254-267.
31.McPherson, R., and Gauthier, A. (2004)
Biochem. Cell. Biol., 82, 201-211.
32.Sato, R., Inoue, J., Kawabe, Y., Kodama, T.,
Takano, T., and Maeda, M. (1996) J. Biol. Chem.,
271, 26461-26464.
33.Marquart, T. J., Allen, R. M., Ory, D. S.,
and Baldan, A. (2010) Proc. Natl. Acad. Sci. USA, 107,
12228-12232.
34.Orekhov, A. N., Andreeva, E. R., Andrianova,
I. V., and Bobryshev, Y. V. (2010) Atherosclerosis,
212, 436-443.
35.Allahverdian, S., and Francis, G. A. (2010)
Trends Cardiovasc. Med., 20, 96-102.
36.Waldo, S. W., Li, Y., Buono, C., Zhao, B.,
Billings, E. M., Chang, J., and Kruth, H. S. (2008) Am. J.
Pathol., 172, 1112-1126.
37.Schwartz, Y. Sh., and Svistelnik, A. V. (2012)
Biochemistry (Moscow), 77, 246-260.