On the Photocycle of 4-Ketobacteriorhodopsin
L. V. Khitrina
Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia; fax: (495) 939-3181; E-mail: khitr@yandex.ru
Received March 14, 2012
The artificial pigment 4-ketobacteriorhodopsin is an interesting analog of bacteriorhodopsin. Arguments concerning the scheme of the photocycle of 4-ketobacteriorhodopsin are discussed.
KEY WORDS: chromophore, retinal analogs, 13-cis-cycle, M-intermediate, M419, K570DOI: 10.1134/S0006297912090076
Abbreviations: BR, bacteriorhodopsin; PM, purple membrane; Z-BR and E-BR, BR with retinal chromophore in 13-cis- and all-trans-configurations, respectively; λmax, maximum of the major band in the absorption spectrum of the chromophore; the lower index of an intermediate of the cycle (e.g. M412, K570) is its differential maximum.
Bacteriorhodopsin (BR) is a light-dependent generator of
Δµ–H+ [1-5]. It is isolated from the bacterium Halobacterium
salinarum (halobium) as purple membranes (PM). The
chromophore of the pigment consists of the Schiff base of retinal and
the ε-amino group of a lysine residue of the protein. The
transfer of H+ occurs during a cyclic transformation of the
pigment (the photocycle, see table). Chromophore modification is an
approach to the study such pigments. The 4-ketoanalog combines high
efficiency and a significant deceleration of the photocycle, and this
is of importance for both scientific and applied problems [12-15]. Unfortunately, the
unsuccessful scheme of the 4-ketoBR photocycle in a series of
publications (table, item 7; [9-11]) leads to delay in discussion of new experimental
data. The purpose of the present work is to show apparent errors in
those publications.
Features of BR and its 4-ketoanalog
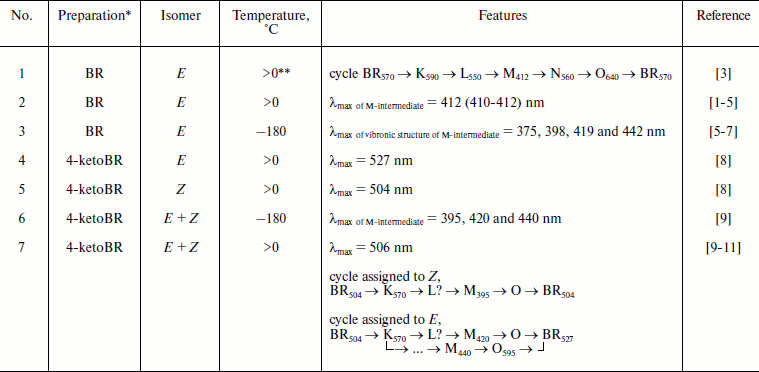
* For BR data are presented for PM, preparations of 4-ketoBR are
membranes resulting on the interaction of apo-membranes and
4-ketoretinal.
** Above 0ºC and below the temperature of the first signs of
thermal denaturation of the preparation (usually below 30ºC).
A group of biophysicists has proposed in 1991 a scheme of 4-ketoBR functioning [9], and the authors defended it later [10, 11], whereas a group from Pushchino supported it also in 2009 in interviews. These authors consider three peaks in the spectrum of M-intermediate of 4-ketoBR detected at –180ºC (table, item 6) to be a specific feature of 4-ketoBR and conclude that these peaks indicate three different M-containing cycles (table, item 7), whereas the 13Z-form enters one of the cycles and the E-conformer is a precursor of two other cycles. The authors of [9-11] include the recorded K570 into all three cycles of the scheme, do not notice the L-intermediate, doubt its presence in 4-ketoBR, and indicate a branch of cycle “shortening” from M to the initial state. However, there is no doubt that on prolonged illumination a rapid return is recorded considering that the photosensitivity of virtually all intermediates has been described long before [5, 16]; in particular, such transition (the so-called “blue inhibition”) is a well-studied phenomenon [16, 17]. Moreover, the photosensitivity of M in the cycle of the analog is not sufficient to indicate the branching M → (analog) BRin steady-state in the dark.
The vibronic structure of the M-intermediate of BR containing the natural retinal in low-temperature spectroscopy is also known [5-7] (table, item 3), but it does not suggest parallel cycles. Moreover, such ideas about the functioning mechanisms became out of date after publication of [2], which established the reversibility of the majority (or all) stages of the BR cycle. Maxima of low temperature peaks of BR and 4-ketoBR are rather close (table, items 3 and 6), and even more peaks have been found in BR (possibly because of increase resolution).
In works [9, 10] the accumulation of a photostationary long-lived M440 is an additional confirmation of the scheme, but phototransformations of intermediates are diverse [5], and most long-lived product will be accumulated, independent of its relation with a single turnover of the cycle.
As differentiated from the proton transfer cycle of E-BR, the Z-cycle under usual conditions has neither an M-intermediate nor H+ transfer, and the life of K-like intermediates is longer [1, 18, 19]. It was shown by Kaulen’s group [20-22] that at high pH the M-intermediate and proton transfer appear in the Z-cycle, but the decay of K accelerates to times typical for the E-cycle. Thus, depending on conditions, BR and its analogs either have in their cycles an M-like intermediate or specific long-lived long-wavelength intermediates [21-24]. We compared the kinetics of spectral transformations of individual forms of E- and Z-4-ketoBR and isomerized preparations containing a mixture of these forms [8], and we found that the E-cycle contains no long-wavelength intermediates, and all signals recorded in this region on uncontrolled preparations are a summary of K-like long-lived intermediates of the Z-cycle and a decrease in the optical density in the main band of the E-cycle specific absorption. Therefore, the assignment of M395 of 4-ketoBR to the Z-cycle under conditions discussed in [9, 10] (table, item 7) and K570 to all cycles of the E- and Z-forms of the analog seems to be unreasonable. An unclear situation with L is caused by a high amplitude of K570 which is specific just for the Z-cycle (a similar case has been analyzed in detail for phenyl analogs of BR [25]). We have recorded similar long-wavelength kinetics in the cycles of individual Z-forms of 4-ketoBR [8] and of other analogs [23-26], but no such kinetics have been recorded in the cycle of the individual E-form of 4-ketoBR [8]. The comparison of λmax (table, items 4, 5, and 7) shows that in preparations of the authors of the scheme of the cycle [9-11] Z-4-ketoBR is prevalent (we have also studied similar mixed preparations [8, 12, 14]). The maximum of the differential spectrum of the E-form K-intermediate is further to the red than that of the Z-form [5, 18]. Therefore, it is reasonable to assign K570 only to the Z-cycle and exclude it from the scheme of the E-4-ketoBR cycle.
Thus, the scheme of the 4-ketoBR proposed and discussed in works [9-11] is not experimentally consistent and contradicts many findings.
REFERENCES
1.Stoeckenius, W., Lozier, R. H., and Bogomolni, R.
A. (1979) Biochim. Biophys. Acta, 505, 215-278.
2.Chernavskii, D. S., Chizhov, I. V., Lozier, R. H.,
Murina, T. M., Prokhorov, A. M., and Zubov, B. V. (1989) Photochem.
Photobiol., 49, 649-653.
3.Kalaidzidis, I. V., Kaulen, A. D., Radionov, A. N.,
and Khitrina, L. V. (2001) Biochemistry (Moscow), 66,
1220-1233.
4.Dioumaev, A. K., and Lanyi, J. K. (2009) J.
Phys. Chem. B, 113, 16643-16653.
5.Balashov, S. P., and Litvin, F. F. (1985)
Photochemical Transformations of Bacteriorhodopsin [in Russian],
MGU Publishers, Moscow.
6.Balashov, S. P., and Litvin, F. F. (1981)
Biofizika, 26, 557-570.
7.Karneeva, N. V., Balashov, S. P., and Litvin, F. F.
(1982) Dokl. Akad. Nauk SSSR, 263, 725-729.
8.Khitrina, L. V., and Lazarova, Ts. R. (1989)
Biokhimiya, 54, 136-139.
9.Brown, L. S., Druzhko, A. B., Lukashev, E. P., and
Chamorovsky, S. K. (1991) Biol. Membr. (Moscow), 8,
460-467.
10.Brown, L. S., Druzhko, A. B., Lukashev, E. P.,
and Chamorovsky, S. K. (1992) Biofizika, 37, 79-85.
11.Druzhko, A. B., and Chamorovsky, S. K. (1995)
Biosystems, 35, 133-136.
12.Drachev, A. L., Drachev, L. A., Evstigneeva, R.
P., Kaulen, A. D., Lazarova, Ts. R., Laikhter, A. L., Mitsner, B. I.,
Skulachev, V. P., Khitrina, L. V., and Chekulaeva, L. N. (1984)
Biol. Membr. (Moscow), 1, 1125-1142.
13.Crouch, R. K. (1986) Photochem.
Photobiol., 44, 803-807.
14.Khodonov, A. A., Eremin, S. V., Lokshin, J. L.,
Shvets, V. I., Demina, O. V., Khitrina, L. V., and Kaulen, A. D. (1996)
Bioorg. Khim., 22, 745-776.
15.Vanhanen, J., Leppanen, V. P., Jaaskelainen, T.,
Parkkinen, J. P. S., and Parkkinen, S. (1999) Opt. Mater.,
12, 473-480.
16.Karvaly, B., and Dancshazy, Z. (1977) FEBS
Lett., 76, 36-40.
17.Dancshazy, Z., Drachev, L. A., Ormos, P., Nagy,
K., and Skulachev, V. P. (1978) FEBS Lett., 96,
59-63.
18.Sperling, W., Carl, P., Rafferty, Ch., and
Dencher, N. A. (1977) Biophys. Struct. Mech., 3,
79-94.
19.Drachev, A. L., Drachev, L. A., Kaulen, A. D.,
Skulachev, V. P., and Khitrina, L. V. (1988) Biokhimiya,
53, 707-713.
20.Zorina, V. V., and Kaulen, A. D. (1988) Biol.
Membr. (Moscow), 5, 1135-1144.
21.Kaulen, A. D., Drachev, L. A., and Zorina, V. V.
(1990) Biochim. Biophys. Acta, 1018, 103-113.
22.Drachev, L. A., Dracheva, S. V., and Kaulen, A.
D. (1993) FEBS Lett., 332, 67-70.
23.Drachev, L. A., Kaulen, A. D., Khitrina, L. V.,
Eremin, S. V., Khodonov, A. A., Shvets, V. I., and Chekulaeva, L. N.
(1993) Biochemistry (Moscow), 58, 545-550.
24.Khitrina, L. V., Drachev, L. A., Eremin, S. V.,
Kaulen, A. D., and Khodonov, A. A. (1992) in Proc. V Int.
Conf.: Structures and Functions of Retinal Proteins (Dourdan)
(Rigaud, J. L., ed.) Vol. 221, Collogue INSERM/John Libbey Eurotext
Ltd., Montrouge, France, pp. 167-170.
25.Drachev, A. L., Zorina, V. V., Mitsner, B. I.,
Khitrina, L. V., Khodonov, A. A., and Chekulaeva, L. N. (1987)
Biokhimiya, 52, 1559-1569.
26.Danshina, S. V., Drachev, A. L., Drachev, L. A.,
Kaulen, A. D., Mitsner, B. I., Khitrina, L. V., and Khodonov, A. A.
(1989) Bioorg. Khim., 15, 307-312.