Mitochondria-Targeted Plastoquinone Derivatives as Tools to Interrupt
Execution of the Aging Program.
5. SkQ1 Prolongs Lifespan and Prevents
Development of Traits of Senescence
V. N. Anisimov1, L. E. Bakeeva2, P. A. Egormin1, O. F. Filenko3, E. F. Isakova3, V. N. Manskikh4, V. M. Mikhelson5, A. A. Panteleeva2, E. G. Pasyukova6, D. I. Pilipenko2, T. S. Piskunova1, I. G. Popovich1, N. V. Roshchina6, O. Yu. Rybina6, V. B. Saprunova2, T. A. Samoylova3, A. V. Semenchenko1, M. V. Skulachev4, I. M. Spivak5, E. A. Tsybul'ko6, M. L. Tyndyk1, M. Yu. Vyssokikh2, M. N. Yurova1, M. A. Zabezhinsky1, and V. P. Skulachev2,4,7*
1Petrov Institute of Oncology, Pesochny pr. 2, 197758 St. Petersburg, Russia2Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119991 Moscow, Russia; fax: (495) 939-0338; E-mail: skulach@belozesky.msu.ru
3Biological Faculty, Lomonosov Moscow State University, 119991 Moscow, Russia
4Mitoengineering Center, Lomonosov Moscow State University, 119991 Moscow, Russia
5Institute of Cytology, Russian Academy of Sciences, Tikhoretsky pr. 4, 194064 St. Petersburg, Russia
6Institute of Molecular Genetics, Russian Academy of Sciences, pl. Kurchatova 2, 123182 Moscow, Russia
7Faculty of Bioengineering and Bioinformatics, Lomonosov Moscow State University, 119991 Moscow, Russia
* To whom correspondence should be addressed.
Received December 29, 2007; Revision received August 15, 2008
Very low (nano- and subnanomolar) concentrations of 10-(6′-plastoquinonyl) decyltriphenylphosphonium (SkQ1) were found to prolong lifespan of a fungus (Podospora anserina), a crustacean (Ceriodaphnia affinis), an insect (Drosophila melanogaster), and a mammal (mouse). In the latter case, median lifespan is doubled if animals live in a non-sterile vivarium. The lifespan increase is accompanied by rectangularization of the survival curves (an increase in survival is much larger at early than at late ages) and disappearance of typical traits of senescence or retardation of their development. Data summarized here and in the preceding papers of this series suggest that mitochondria-targeted antioxidant SkQ1 is competent in slowing down execution of an aging program responsible for development of age-related senescence.
KEY WORDS: SkQ1, antioxidants, mitochondria, aging, senescence, therapyDOI: 10.1134/S0006297908120055
Abbreviations: C12TPP, dodecyltriphenylphosphonium; MitoQ, 10-(6′-ubiquinonyl) decyltriphenylphosphonium; PQ, decyl plastoquinone; ROS, reactive oxygen species; SkQs, cationic derivatives of plastoquinone or methyl plastoquinone; SkQ1, 10-(6′-plastoquinonyl) decyltriphenylphosphonium; SkQR1, 10-(6′-plastoquinonyl) decylrhodamine 19; TPP, tetraphenylphosphonium.
As shown in the preceding papers [1-5], cationic derivatives of plastoquinone (SkQs):
- easily penetrate through lipid membranes, generating the theoretically expected diffusion potential [2];
- operate as antioxidants and prooxidants in isolated mitochondria, the window between the anti- and prooxidant activities being very large [2];
- in cells cultures, specifically accumulate inside mitochondria and prevent H2O2-induced apoptosis as well as light-induced necrosis in the presence of a photosensitizer [2];
- in animals, lower levels of age-induced oxidation of lipids and proteins [5];
- show favorable therapeutic effects on age-related diseases such as heart infarction [3], heart arrhythmia [3], stroke [3], kidney ischemia [3], some kinds of cancer [4], eye pathologies (cataract, retinopathies, glaucoma, and uveitis [5]), and osteoporosis [5].
In this work, we investigated effect of 10-(6′-plastoquinonyl) decyltriphenylphosphonium (SkQ1) on lifespan of fungi, invertebrates, and mammals. It was found that SkQ1 at extremely low concentrations operates as a geroprotector increasing the lifespan and preventing development of a number of typical traits of aging. It looks like SkQ1 slows down execution of a senescence program so that aging results in death later than without SkQ1 due to absence (or a strong reduction) of the senescence stage.
MATERIALS AND METHODS
Podospora anserina. Mycelia of fungus P. anserina were derived from a wild-type strain [6]. Medium M2 was used as a minimal synthetic growth medium [6]. Juvenile cultures were derived from a mononuclear ascospore incubated on germination medium G as described by Borghouts et al. [7]. The effect of SkQ1 on P. anserina growth and longevity was measured on mycelia grown in the M2 medium on Petri dishes. The M2 medium (1 liter) contained KH2PO4, 0.25 g; K2HPO4, 0.3 g; MgSO4⋅7H2O, 0.25 g; urea, 0.5 g; dextran, 5 g; biotin, 5 µg; thiamine, 50 µg; citric acid, 2.5 mg; ZnSO4⋅7 H2O, 2.5 mg; CuSO4⋅5 H2O, 0.125 mg; Fe(NH4)(SO4)2⋅12 H2O, 0.25 mg; MnSO4⋅H2O, 25 µg; H3BO4, 25 µg; NaMoO4⋅2 H2O, 25 µg; agar, 12.5 g; pH 6.5. Ethanol solutions of SkQ1 or SkQR1 (10-(6′-plastoquinonyl) decylrhodamine 19) were added to the molten M2 medium at 40-50°C. At zero time, a small piece of the P. anserina mycelium was transferred from the front of a juvenile culture to a medium-containing 90 mm Petri dish. Every two days, a small inoculum from the growing mycelium front was transferred to another Petri dish. Mycelia were grown at 27°C in the dark. Four inocula were simultaneously grown on each Petri dish. Growth of 16 colonies obtained from a single spore was studied in each experiment.
Ceriodaphnia affinis. Crustacean invertebrates C. affinis were kept living in the medium from a 100-liter aquarium with a stationary biocomplex (pH 7.8). Suspension of Chlorella vulgaris was used as the food. Two C. affinis individuals of age =24 h were placed into a flask containing 30 ml medium. Each group differing in the SkQ1 concentration was composed of 20 animals. Temperature was about 22°C. The medium was changed each second day. SkQ1 was added as an ethanol solution, the maximal amount of ethanol in the growth medium being 0.001 ml/liter.
Drosophila melanogaster. The isogenic D. melanogaster w1118 line was used in all experiments. The flies were maintained on a wheat-sugar-raisin medium at 25°C.
Fresh 20 mM stock solutions of studied quinones, C12TPP (dodecyltriphenylphosphonium), and MitoQ (10-(6′-ubiquinonyl) decyltriphenylphosphonium) in 96% ethanol were prepared for each experimental trail and stored at -20°C. Working solutions were diluted with distilled water, being prepared ex tempore. We used 20 µM, 20 nM, 20 pM, and 20 fM SkQ1 solutions and SkQ1-free medium as a control in the pilot experiment for choosing the working concentration of compounds under study. In all following experiments, 20 pM solutions of the studied compounds were used. As a control, 96% ethanol diluted 109 times was used (ethanol concentration in working solutions of the studied compounds). In each case, 0.1 ml of the solution was applied to the medium surface and dried overnight at 25°C.
Five virgin females or males collected from parental vials were placed in a vial containing culture medium treated with SkQs, their analogs, or control solution. Flies were transferred to fresh medium with appropriate solution once a week. Dead flies were counted every day. Sample sizes per treatment varied from 50 to 425 flies. For comparisons of survival curves, the Kaplan-Meier logrank test was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, USA).
Electron microscopy of flight muscles of D. melanogaster was performed as in [3].
SHR and HER-2/neu mice. Females of outbred SHR mice or transgenic FVB/N strain female mice bearing human oncogene HER-2/neu related to breast cancer (received from the Italian National Center for Research on Aging, Ancona, Italy) were used in the experiments. The animals received a standard PK-120 diet ad libitum [8] and were kept in a non-sterile vivarium at room temperature and standard light regime. SkQ1 (0.5-2500 nmol/kg per day) was added to the drinking water every day starting from two months of age. The control animals received pure drinking water. The changes in body mass and consumption of food and water were tested monthly. Estrual cycles were followed every three months as described [9]. Parameters of longevity were calculated (median, mean, and maximal lifespan). Post mortem complete autopsy was performed for histological analysis of tumors and non-tumor pathologies [10]. GraphPad Prism version 5.00 software (GraphPad Software) was used for statistical calculations. Student's t-test and χ2 Fisher's criteria were employed for analysis.
Fibroblasts from the mice treated with SkQ1. Subcutaneous fibroblasts were isolated from tails of the mice receiving SkQ1 with drinking water or of control animals. A piece of tail (1.5 cm) was placed in a tube with DMEM (Dulbecco's Modified Eagle's Medium) (Gibco, USA) supplemented with 200 IU/ml penicillin, 0.2 mg/ml streptomycin, and 0.05 mg/ml fungizone. After mincing, tissue was transferred to DMEM medium with 16% fetal calf serum, 0.3 mg/ml glutamine, and the same antibiotics diluted twice. The fibroblasts leaving tissue pieces in 3-5 days were grown for 10-12 days, removed with trypsin and EDTA, and reseeded to prepare monolayer culture. Apoptosis was induced with addition of H2O2 [2]. Activity of β-galactosidase was detected after staining with X-Gal (1 mg/ml, pH 6.0) overnight at 37°C. Phosphorylation of histone H2AX was determined by immunostaining of the cells with monoclonal antibodies to the phosphorylated protein (Upstate, USA) and secondary rabbit anti-IgG conjugated with fluorophore Texas Red (Vector Labs, USA).
RESULTS
SkQ1 increases lifespan of mycelia of the fungus Podospora anserina. During the last decade, the fungus P. anserina has become a subject of interesting experiments on regulation of lifespan. In particular, it was found that duration of life of this short-lived organism can experimentally be significantly changed by affecting mitochondrial respiration, morphology, and reactive oxygen species (ROS) formation [11, 12]. This is why we started the gerontological part of our SkQ project with studies on P. anserina.
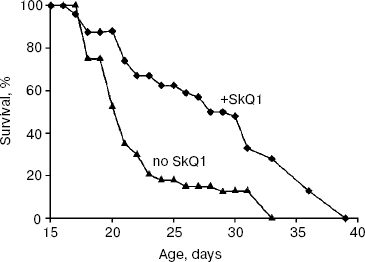
As experiments showed (Fig. 1), low (100 nM) SkQ1 added to the agar medium increases the P. anserina lifespan. SkQ1 proved to be more effective at early stages of aging. Thus, on day 21, 70% of the fungi were dead without SkQ1 and only 25% with SkQ1. The median lifespan was increased by SkQ1 by 50%. The difference decreased with age so that lifespan of the last 10% of fungi was increased by only 20% (Fig. 1). Higher (1 µM) and lower (20 nM) SkQ1 concentrations were less effective than 100 nM. The SkQ1 effect was accompanied by retardation of development of such a trait of mycelium aging as disappearance of its protrusion to the air phase (not shown in the figures).
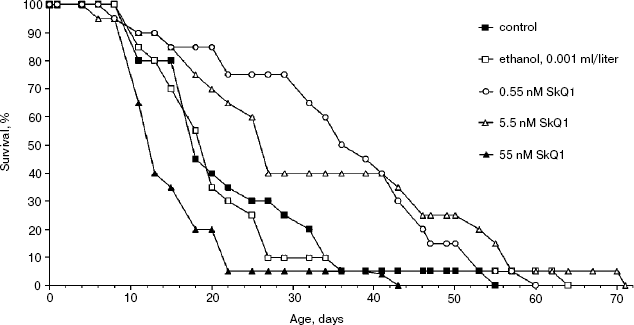
Effect of SkQ1 on lifespan of Ceriodaphnia affinis. The crustacean C. affinis is a convenient subject for lifespan research since its imago life cycle is usually as short as 15-20 days and cultivation under laboratory conditions is not a problem. One more advantage is that the concentration of SkQ1 affecting the living subject could be exactly estimated since C. affinis were living, in fact, in the water solution of SkQ1 of a known dilution. A disadvantage of this species is the genetic heterogeneity of its population, which can affect the results. Figure 2 shows that 5.5 and 0.55 nM SkQ1 increased the C. affinis lifespan, whereas 55 nM SkQ1 had an opposite effect. The median lifespan at optimal (0.55 nM) SkQ1 concentration was doubled compared with samples without SkQ1.Fig. 1. Effect of 100 nM SkQ1 on lifespan of the fungus Podospora anserina. For conditions, see “Materials and Methods”.
Drosophila melanogaster lifespan and age-dependent mitochondrial ultrastructure changes are affected by SkQ1. Drosophila melanogaster is an effective model system for gerontological studies due to the possibility to use genetically well characterized lines, including isogenic wild type and mutant flies, and short lifespan as compared to vertebrates. Our experiments showed that SkQ1 prolonged the lifespan of flies even though the effect was not as strong as for C. affinis.Fig. 2. Effect of various concentrations of SkQ1 on lifespan of the crustacean Ceriodaphnia affinis. For conditions, see “Materials and Methods”.
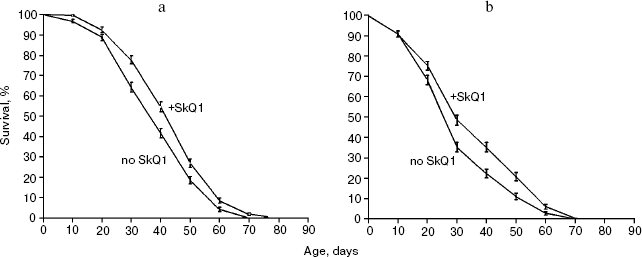
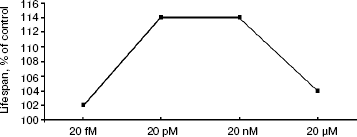
Survival curves (Fig. 3) demonstrate that 20 pM SkQ1 was effective in females (a) as well as in males (b); survival curves for flies of both sexes receiving SkQ1 were significantly different from survival curves of control flies (p < 0.0001 in both cases). Maximal effect was revealed between 20 pM and 20 nM SkQ1 (Fig. 4).
Fig. 3. SkQ1 (20 pM) increases the lifespan of D. melanogaster females (a) and males (b). Totally 425 flies per sex per treatment were analyzed. Survival curves of females and males receiving SkQ1 are significantly different from control curves (p < 0.0001 in both cases).
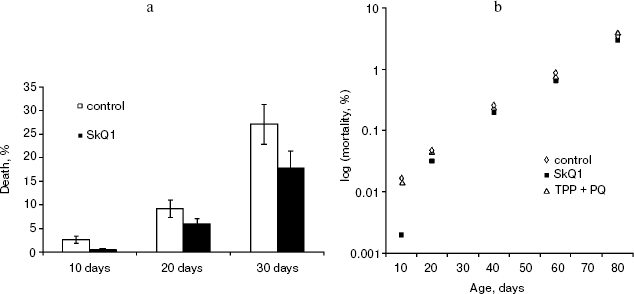
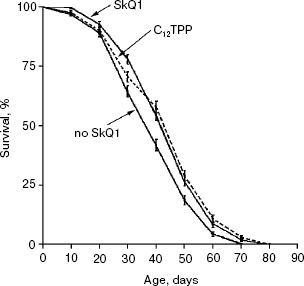
Detailed analysis of the effect of SkQ1 on lifespan of females revealed that it is more pronounced at early stages of life. As seen in Fig. 5a, mortality during the first 10 days of life was almost completely abolished by SkQ1, whereas during the first 20 days SkQ1 decreased mortality by only 30%. At greater ages, the effect of SkQ1 became even smaller. This phenomenon is also illustrated by Fig. 5b where mortality rate is presented in half-logarithmic coordinates as a function of age. It is evident that the effect of SkQ1 was at its maximum for the first 10 days (here mortality was decreased by one order of magnitude). A mixture of TPP (tetraphenylphosphonium) and PQ (decyl plastoquinone), the two constituents of SkQ1, was completely ineffective (at least at such low concentration as was used in the case of SkQ1). Solution of C12TPP (20 pM), a compound containing two additional methylene groups instead of plastoquinone in SkQs, was also found to increase the lifespan of females. However, substantial difference in effects of SkQ1 and C12TPP was revealed. SkQ1, as already mentioned, primarily affected young females, whereas C12TPP was practically ineffective in young flies but effective later in life. As a result, the C12TPP survival curve followed the control curve during the first 20 days and the SkQ1 curve during the last 40 days (Fig. 6). As was recently found in our group [13], hydrophobic cations such as SkQs and C12TPP can operate as carriers of fatty acid anions, mediating in this way uncoupling of oxidative phosphorylation by these acids. This, in turn, should decrease ROS production not only by Complex I but also by Complex III [14]. In this context, it is worth noting that direct antioxidant effect of SkQ1 carried out by its quinol residue should be related to those ROS that are generated by Complex I inside mitochondria rather than to ROS produced by Complex III close to the outer surface of the inner mitochondrial membrane [1]. Thus, comparison of the SkQ1 and C12TPP effects on longevity of flies seems to indicate that ROS increasing the early and the late life mortality risks are generated by different mechanisms.Fig. 4. Dependence of D. melanogaster mean lifespan on SkQ1 concentration. Totally 50 females per concentration were analyzed.
Fig. 5. SkQ1 prolongs lifespan mainly at early stages of life: cumulative percent of death (a) and mortality rate (b). 20 pM SkQ1 or mixture of 20 pM TPP and 20 pM PQ were employed. Totally 425 females per treatment were analyzed.
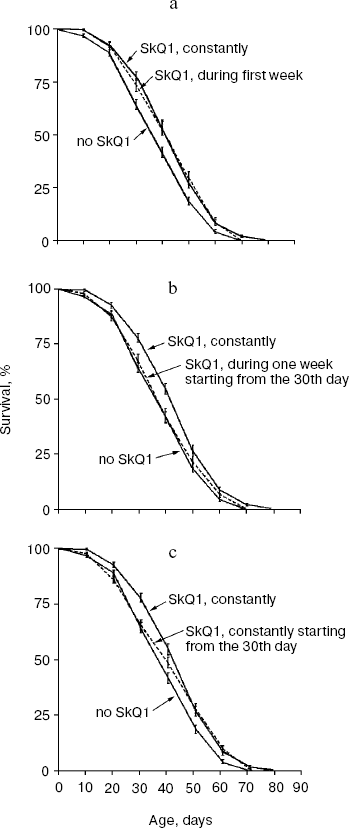
Figure 7 summarizes results of experiments when the SkQ1 treatment was carried out on certain stages of aging rather than during the entire life. Figure 7a shows that the survival curve of flies receiving SkQ1 during the first week of life did not differ from the survival curve of flies receiving the compound constantly up to death (p = 0.75), whereas both were significantly different from the control curve (p < 0.0004 and p < 0.0001, correspondingly). Such a short-term SkQ1 treatment proved to be ineffective when started on the 30th day (Fig. 7b). However, constant administration of SkQ1 from the 30th day up to the end of life was quite effective (Fig. 7c), and the survival curve of treated females in this case was significantly different from the control curve (p = 0.0025).Fig. 6. A 20 pM C12TPP (an SkQ1 analog lacking the quinone part) increases lifespan of D. melanogaster females primarily at the late stage of aging. Totally 425 females per treatment were analyzed. C12TPP and SkQ1 survival curves are significantly different from control curve (p < 0.0001 in both cases).
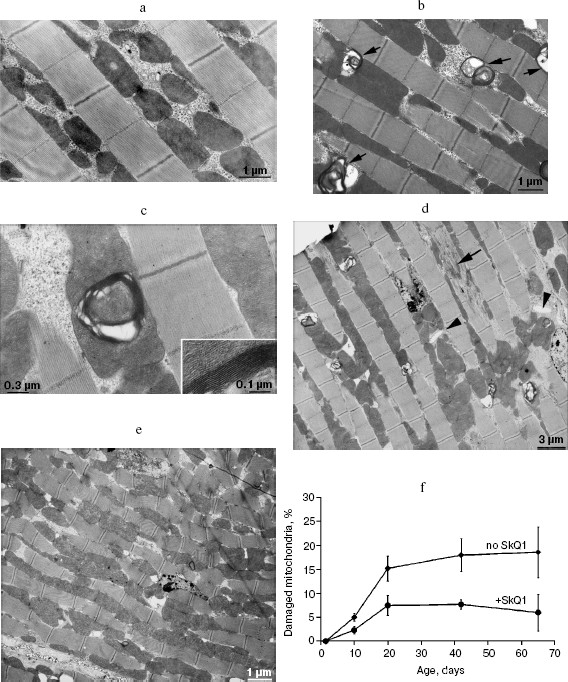
Figure 8 shows the effect of SkQ1 on mitochondrial ultrastructure in flight muscles of D. melanogaster individuals of different ages. One can see that aging is accompanied by appearance of organelles of dramatically changed structure (membrane multilayers resembling myelin) called “mitochondrial swirls” [15]. Such structures were absent from newborn (1.5 day) flies but appear as early as on the 10th day of life (cf. Figs. 8a and 8b; see Fig. 8c for multilayer structure at higher magnification). The “mitochondrial swirls” were usually integrated into chains of mitochondria localized between bundles of actomyosin filaments. In the SkQ1-treated flies, changed mitochondria appeared much less frequently on the 10th and 20th days. Even on the 65th day (when in the absence of SkQ1 damaged actomyosin filaments appear, Fig. 8d) these structures (“mitochondrial swirls”) are still very rare (Fig. 8, e and f).Fig. 7. Administration of 20 pM SkQ1 during the first week of life (a) and constantly starting from the 30th day (c) increases lifespan of females as does 20 pM solution of SkQ1 during the entire life, whereas SkQ1 administered during one week starting from the 30th day is ineffective (b). From 225 (a, b) to 425 females (c) per treatment were analyzed. In Fig. 7a and Fig. 7c, survival curves of SkQ1-treated females are significantly different from control curves (p < 0.0004 and p = 0.0025, correspondingly).
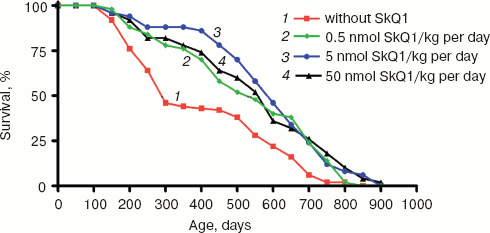
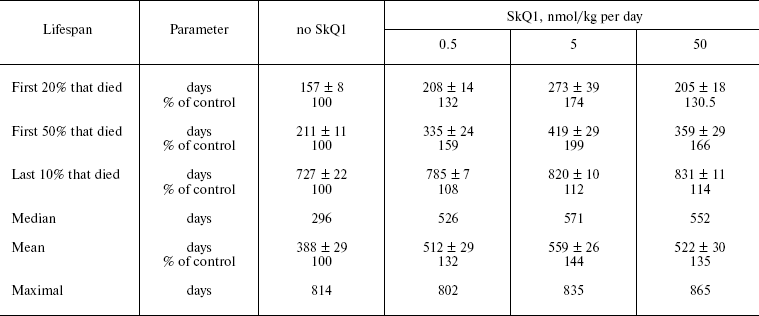
SkQ1 prolongs lifespan of mice and retards appearance of senescence traits. Two experiments were performed to reveal effect of SkQ on the lifespan of outbred SHR mice. One of them was started in December, 2004, and another in June, 2005. In each experiment, there were four groups of females (25 animals per group)--one for control mice (not treated with SkQ1) and three other for mice receiving with drinking water 0.5, 5, or 50 nmol SkQ1/kg per day. The results of the two experiments are summarized in Fig. 9 (see color insert). One can see that all the SkQ concentrations used significantly prolonged the lifespan, and the middle (5 nmol) concentration seems to be optimal. Moreover, like in the above-described experiments on fungi and invertebrates, the effect was especially strong during initial and middle stages of aging rather than on maximal lifespan, which increased only slightly (rectangularization of the survival curves). It is remarkable that lifespan of the first 50% of animals that died (median lifespan) in fact doubled with the optimal SkQ1 dose. For mammals, such a strong shift of the survival curve to the right is very rare. On day 300, more than 50% of control mice had already died, whereas in the group receiving 5 nmol SkQ1 almost all animals were still alive. These relationships are illustrated by Table 1.Fig. 8. Effect of SkQ1 on mitochondrial age-related ultrastructural changes in D. melanogaster flight muscles. a) 1.5-day-old fly; b) 10-day-old fly; c) 52-day-old fly; d, e) 65-day-old flies. e) Flies received 1.85 nM SkQ1; b) arrows designate damaged mitochondria (“mitochondrial swirls”); d) arrows designate damaged actomyosin filaments; arrowheads show tracheoles; f) 1.85 nM SkQ1 prevents appearance with age of “mitochondrial swirls” (males).
Table 1. Effect of SkQ1 on lifespan of SHR miceFig. 9. SkQ1 increases lifespan of mice. The figure summarizes results of two experiments on 200 females. In each experiment, four groups of females (25 mice per group) were studied.
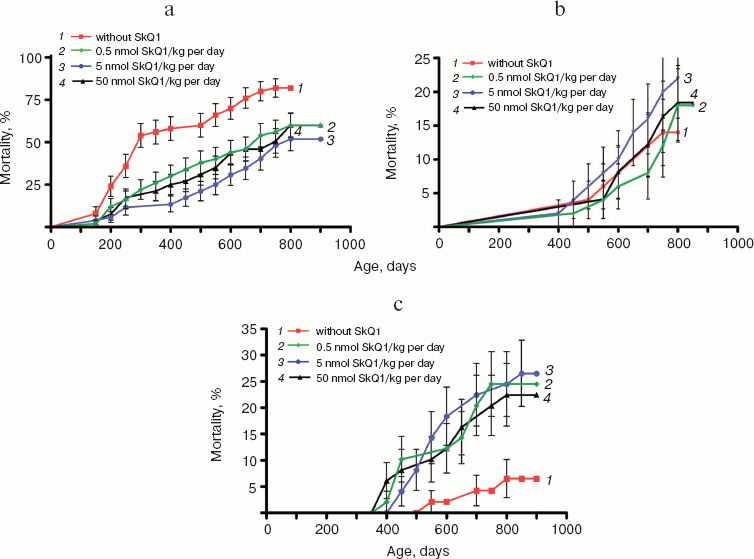
Analysis of the causes of death revealed that the SkQ-induced increase in the lifespan is mainly due to strong lowering of rate of death caused by diseases other than cancer (Fig. 10a; see color insert). Most of these were infections, such as pneumonia, hepatitis, nephritis, colitis, etc., that proved to be a consequence of the fact that mice were living under non-sterile conditions. This made it possible to take into account probable preventive effect of SkQ1 on an age-dependent decline of the immune system (see “Discussion”). SkQ1 was without statistically significant effect on the death of SHR mice caused by cancers other than mammary gland adenocarcinomas (Fig. 10b; see color insert).
As shown in Fig. 10c (color insert), there is a specific kind of cancer for which the probability increases if mice received SkQ1. This is mammary gland adenocarcinoma. However, further analysis revealed that such an unfavorable effect is a consequence of a favorable one, namely prolongation by SkQ1 of estrual function, rather than of any carcinogenic activity of our compound.Fig. 10. Age-dependent mortality of mice (a) from causes other than tumors, (b) due to cancer other than mammary gland adenocarcinoma, and (c) due to mammary gland adenocarcinoma.
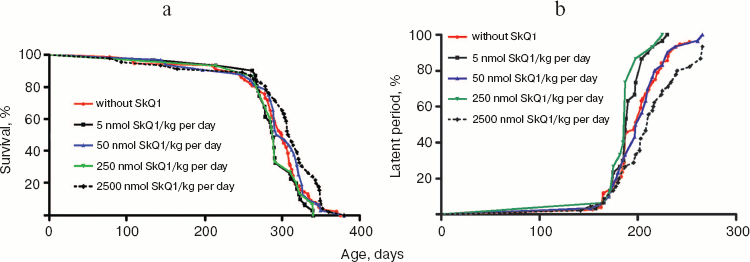
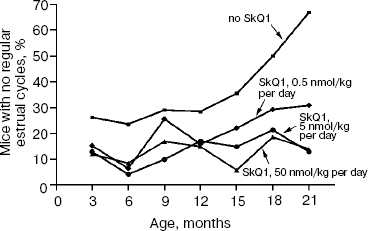
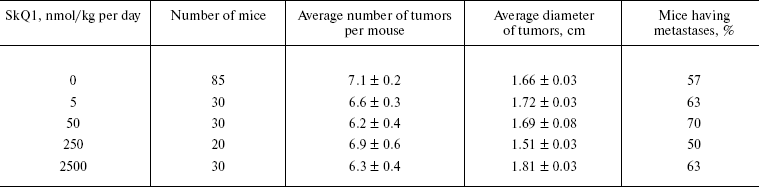
The above problem was studied on two groups of mice, i.e. outbred SHR and transgenic HER-2/neu. In the latter case all the mice died due to development of the mammary gland adenocarcinomas at age of about one year. As one can see in Fig. 11a (color insert) and Table 2, SkQ1 is without measurable effect on longevity of the HER-2/neu mice. Essential parameters of carcinogenesis were not affected by any concentrations of SkQ1 studied (Fig. 11b, color insert, and Table 3). However, SkQ1 completely prevented disappearance of regular estrual cycles in the outbred SHR mice (Fig. 12). Thus, most probably mammary gland adenocarcinoma could not develop in the great majority of the control mice, first of all, due to age-dependent mammary gland involution [16].
Table 2. SkQ1 does not affect the lifespan of HER-2/neu mice dying due to mammary gland adenocarcinomaFig. 11. SkQ1 does not influence development of mammary gland adenocarcinomas in HER-2/neu mice. Survival (a) and latency period in the tumor development (b) as functions of age.
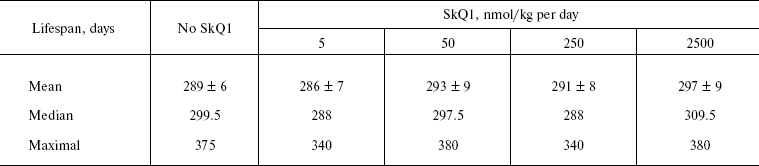
Table 3. SkQ1 does not affect development of
mammary gland adenocarcinoma in HER-2/neu mice
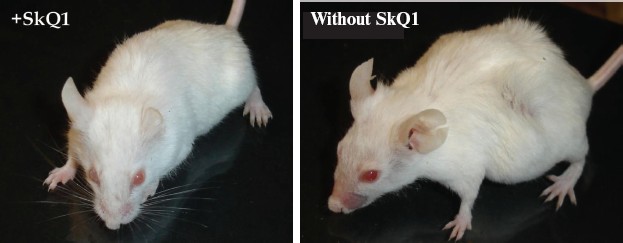
Prolongation of the estrual cycles is not the only geroprotector effect of SkQ1. In Fig. 13 (color insert), photographs of two 630-day-old female SHR mice, one receiving a minimal dose of SkQ1 (0.5 nmol/kg per day) and another without SkQ1, are presented. It seems obvious that the mouse without SkQ1 is in bad shape compared with that with SkQ1. She has lost whiskers and shows obvious traits of baldness and lordokyphosis. For “no SkQ1 mice”, torpor and a body temperature decrease during the last weeks of life were observed. All these traits of senescence were absent in the SkQ1 mice. As to food and water consumption and body weight, they did not significantly differ in all the mouse groups studied (not shown in figures and tables).Fig. 12. SkQ1 prevents disappearance with age of regular estrual cycles in mice.
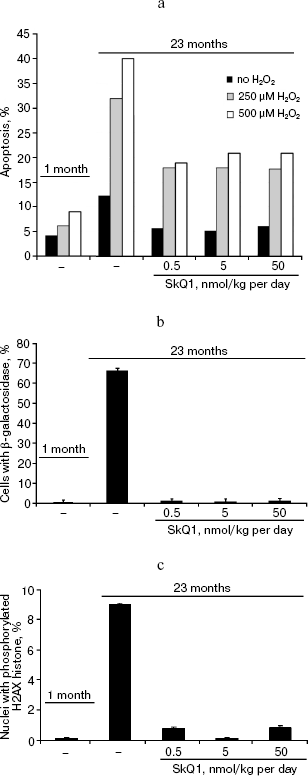
In the last series of experiments on the SHR mice, fibroblasts obtained from the tail of the control and SkQ1 mice of various ages were studied. It was found that spontaneous apoptosis of fibroblasts from old control mice occurs three times more often than in young animals. This difference disappeared if mice received 0.5, 5, or 50 nmol SkQ1/kg per day. Fibroblast from mice that received SkQ1 retained the ability to activate apoptosis by added H2O2, but the effect of H2O2 was not so dramatic as in fibroblasts from untreated mice of the same age (Fig. 14a). It was also shown that the in vivo SkQ1 treatment prevents development of such typical traits of aging as appearance of β-galactosidase activity and phosphorylation of histone H2AX (Figs. 14b and 14c, respectively).Fig. 13. A 630-day-old mice receiving (left photo) and not receiving (right photo) 0.5 nmol SkQ1/kg per day.
Fig. 14. Some properties of fibroblasts obtained from tails of mice receiving or not receiving SkQ1. a) Spontaneous and H2O2-induced apoptosis; b) percentage of β-galactosidase-positive cells; c) percentage of cells with phosphorylated histone H2AX.
DISCUSSION
Quite recently, Longo and coauthors wrote “Caloric restriction is the only non-genetic intervention known to slow down aging and extend lifespan in organisms ranging from yeast to mice” [17]. Data presented in this paper indicate that SkQ1 is an alternative factor prolonging life ranging from a fungus to a mammal. In fact, the mycelial fungus Podospora anserina, invertebrates Ceriodaphnia affinis and Drosophila melanogaster, and mice were found to respond with a significant increase in the median lifespan to the SkQ1 addition to the growth medium, food, or drinking water. For D. melanogaster, the effect was rather small (about 15%) but statistically significant (Fig. 4). For the three other organisms studied, it was much larger, reaching 100% in mice receiving optimal SkQ1 concentration (Figs. 1, 2, and 9). At least for mice, its effect is not related to caloric restriction, since SkQ1 was without any influence on the amount of food consumed. Among geroprotectors, SkQ1 is proved to be a unique small molecule not only due to such a wide range of organisms where it is effective, but also at extremely low values of the concentration required to prolong life. For instance, in the Ceriodaphnia experiments, the optimal SkQ concentration in the growth medium was 0.55 nM. For Drosophila, it was sufficient to put three drops of 20 pM (2⋅10-11 M) SkQ1 solution on the surface of the food once per week (or even on the first week of life only) to obtain maximal effect. In mice receiving SkQ1 with drinking water, the optimal SkQ1 concentration was 5 nmol/kg per day, 0.5 nmol being still of significant effect. An explanation of such a high efficiency of SkQ1 may be that it is specifically concentrated only in one place of the cell, i.e. in the inner leaflet of the inner mitochondrial membrane, where SkQ1 concentration can reach 0.1 mM when extracellular SkQ1 concentration is as low as 1 pM [1, 2].
The idea that mitochondrially-targeted quinones might be used as geroprotectors was introduced by Murphy and coworkers [18] and simultaneously by one of the authors of the present paper [19]. In Murphy's group [20], an attempt was undertaken to prolong with MitoQ the lifespan of D. melanogaster. No geroprotector effect was observed on wild-type flies, but MitoQ proved to be effective on females of a short-lived mutant that was deficient in mitochondrial superoxide dismutase and showed several traits of progeria [21]. We also tried MitoQ and observed some geroprotective effect even in wild type flies (data not shown in figures). The problem in observing such a phenomenon is that it is large at young ages only. As to the mean and median lifespans, they respond to the studied compounds by rather small increase in these parameters, and the result appears to be statistically significant if the number of flies studied is sufficiently high. It seems possible that for such a post-mitotic organism as a fly, the mean lifespan is not a sensitive trait to reveal potential geroprotectors. It is remarkable that the geroprotecting effect of SkQ1 was much larger when the number of damaged mitochondria (“mitochondrial swirls”) was measured (cf. Figs. 3 and 8f).
The geroprotective effects of SkQ1 could be accounted for within the framework of alternative concepts considering aging as a result of (i) accumulation with age of occasional injuries and (ii) operation of a specific genetic program of slow suicide (for reviews, see [22, 23]). In the former case, SkQ1 simply “cleans the dirtiest place in the cell”, removing from the mitochondrial interior those ROS that are responsible for age-related damage [19]. In the latter case, SkQ prevents execution of an aging program, which slowly kills organisms by means of intramitochondrial ROS, the level of which increases with age in a program-controlled fashion [22, 23]. At present, we cannot discriminate between these two possibilities. However, there are several indirect but independent pieces of evidence making the program scheme more probable.
1. In experiments with D. melanogaster, we found that SkQ1 treatment during first week of life has the same geroprotector effect as treatment during the whole life (Fig. 7a). This result is, in fact, in line with observations that: (i) two days caloric restriction of the fruit fly prolongs the fly's lifespan as effectively as caloric restriction during the whole life [24], and (ii) just the odor of the food decreased the caloric restriction effect upon lifespan of D. melanogaster [25].
These observations can be explained much easier by a reset of the “ontogenetic clock” controlling the aging program than by assuming that SkQ1 or caloric restriction directly prevent accumulation of ROS-induced (or any other) damages in tissues during the whole life. In this context, one might suggest that the age-controlling “ontogenetic clock” system operates in a mitochondrial ROS-dependent fashion so that the target of the geroprotector SkQ1 action is, in fact, mitochondria of the clock cells or any other cells required to execute the clock signal in the tissues.
2. ROS-induced injuries are known to be accumulated in especially large quantities at late ages. Therefore, one might expect that SkQ1 would be more effective near the end of life. In our experiments, we observed, in fact, quite opposite relationships. SkQ1 prevented age-dependent death at early and middle stages of aging, being efficient only slightly in increasing the maximal lifespan (see Figs. 1, 2, 5, and 9). This observation fits the hypothesis that the aging program is a mechanism increasing “evolvability” (the rate of evolution). It was assumed that aging is needed to facilitate natural selection to recognize small but useful traits. These traits can be insufficient for a strong young organism but becomes essential when its functions decline with age [26-29]. Very old organisms compose a small part of the population, and their reproductive potential is already lowered so that their contribution to evolvability should be limited. This means that the aging program becomes unnecessary in such organisms and therefore no longer takes part in the senescence process.
3. Last but not least, one should take into account that the suggestion concerning SkQ1 as a geroprotector was a consequence of the entire basic concept of our project. The hypothesis on programmed aging predicted the main results described in this series of papers. In such a complicated science as biology, the very fact that a hypothesis predicts results is always a strong argument in favor of its validity.
Within the framework of the concept considering aging as an evolvability-increasing invention of living organisms, it seems obvious that many functions of vital importance should decline with age. This means that evolution of all of them is accelerated by aging. This is why there are numerous senescence processes, which after all cause the death of the organism. As to non-aging organisms, they usually die due to a single and always the same reason, and their life is not accompanied by decline of different physiological functions. Thus, pearl mussels, organisms having, in fact, no enemies, live more than two centuries and die because the shell becomes too heavy to be maintained in a vertical position. As a result, death occurs because of lack of food. Not a single trait of senescence was observed. In particular, reproductive potential increases with age since those organisms are growing during their whole lives.
In 2007 Lambert et al. [30] reported that the lifetime of mammals and birds is longer the lower is the rate of H2O2 generation by heart mitochondria during the reverse transfer of electrons from succinate through Complex I (animals of 12 species very differently systematically positioned were studied: from mice to cattle and baboons and from quails to pigeons). No correlation was found when H2O2 generation was measured under forward electron transfer (through both Complex I and Complex III). The longest-living African rodent, the naked mole-rat, seems to be the only exception to this rule. This creature of mouse size is famous for its lifespan of about 28 years instead of the mouse's 2.5-4 years. Cancer, atherosclerosis, immunodeficiency, and even several kinds of pain are unknown for this animal. The queen and her several sexual partners, the only reproducing members of the large community composed of 200-300 soldiers, have no enemies. In the laboratory, naked mole-rats die at the age of about 25-28 for an unknown reason. As was recently shown by Buffenstein, their mortality rate does not depend on age [31], as if the aging program is absent in this animal. The program seemed to be switched off somewhere after ROS because both the rate of ROS generation on the reverse transfer of electrons [30] and the level of biopolymer oxidation [32, 33] in naked mole-rats are higher than in mice. The latter finding is not surprising if one takes into account that activities of superoxide dismutase and catalase in these two species are of the same order of magnitude, and the third main antioxidant enzyme, glutathione peroxidase, is 70-fold less active in mole-rats than in mice [34]. The key for understanding this paradoxical situation seems to be presented by the observation that even very high concentrations of H2O2 failed to induce apoptosis in the mole-rat's cells [35]. In this respect, the mole-rat is reminiscent of long-living mouse with mutations in genes encoding the protein p66shc [36], the enzyme of CoQ synthesis (mClk1) [37], or elongation factor 2 kinase [38], whose cells are also resistant to apoptogenic action of hydrogen peroxide [36, 38] or the prooxidant menadione [37].
A possible mechanism explaining how a function, which became unnecessary for the naked mole-rats, could disappear during evolution was recently described by Park et al. [39]. It was found that these rodents lack an 11 amino acid peptide called substance P, which operates in other animals as mediator of certain kinds of pain (in particular, the pain caused by capsaicin, a compound from capsicum). Capsaicin, like several other pain-inducing factors, does not induce pain-related behavior in the naked mole-rat. The capsaicin sensitivity was restored when a herpes-containing DNA construct encoding the substance P-synthesizing protein was injected into a leg of the animal. The treatment did not affect pain sensitivity of the non-treated legs. Most probably, the naked mole-rat queen and her husbands are so perfectly defended in the center of their underground labyrinth by the army of soldiers, that external pain-causing events are practically excluded. As a result, a mutation making impossible transmission of the pain signal proved to be neutral and therefore avoid elimination by the natural selection mechanism. The same apparently happened with program of aging, which according to our hypothesis requires enemies to operate as an evolution-accelerating mechanism.
It is of importance that SkQ1 concentrations increasing lifespan seem to abolish the aging-induced stimulation of apoptosis rather than completely arrest any ROS-linked apoptotic events (see Fig. 14a). This is why doses of SkQ1 used in our experiments on mice did not decrease antitumor defense, which includes apoptosis. In fact, SkQ1, like another antioxidant, N-acetyl cysteine, prolonged lifespan of a mouse strain prone to lymphomas [4]. SkQ also decreased probability of tumorigenesis in experiments on normal mice if we deal with development of tumors other than mammary gland adenocarcinoma (Fig. 10b, see color insert). As this figure shows, probability of such cancers is not increased in the SkQ1-receiving mice in spite of the fact that these mice lived longer, being much more resistant to numerous infections. This should increase the rate of death caused by other reasons, including cancer, provided that cancer is not retarded by SkQ1. Since such an increase was not observed, one can conclude that SkQ1 possesses certain anticancer activity not only in the lymphoma-prone but also in normal mice. The mammary gland adenocarcinoma is a special case. As is clear from Fig. 12, SkQ1 prevents the age-dependent decline of estrual function, which should, in turn, prevent involution of mammary glands [16]. Such involution for sure results in a situation when mammary gland cancer appears to be a rarer reason for death in control mice compared with those who receive SkQ1. In line with the above reasoning, we found that SkQ1 does not stimulate the development of cancer in the mammary gland adenocarcinoma-prone mice (Fig. 11, color insert, and Tables 2 and 3).
It is remarkable that SkQ1 strongly decreases the death risk for reasons other than cancer (Fig. 10a, see color insert). The reasons in question were numerous infections inevitable in the non-sterile vivarium where the above experiments were carried out. This allowed us to reveal SkQ1 as a tool arresting development of such a typical feature of aging as decline of the immune defense of an organism. Such a decline is due to numerous factors including decrease in number of the antigen-presenting dendrite cells, impairment of chemotaxis and phagocytosis of these cells and neutrophils, failure of dendrite cells to properly stimulate naive CD4+ T cells, some functional injuries in monocytes, decrease in the production and proliferation of normal killer cells, etc. [40]. Importantly, a decline of the immune system represents one of the earliest traits of aging, starting in humans between 10 and 15 years of age [41].
One of the reasons for decline of the immune system during aging is related with age-dependent involution of thymus, the major source of T-cells [42]. Progeria in OXYS rats is accompanied with more rapid decrease of the mass, volume, and cellularity of thymus than in normal rats (Obukhova et al., paper in preparation). The right lobe of the thymus in OXYS rats contained 2 times fewer cells than in Wistar rats at 3.5 month of age. Treatment with 250 nmol SkQ1/kg per day resulted in 2.5-fold increase of this number in OXYS rats, while in Wistar rats the increase was only 25%, so this parameter became equal in the two strains of rats. A similar effect of SkQ1 was observed when the area of lymphoid follicles in the spleen (the major source of B-cells) was analyzed.
In our preceding paper [1], we listed 20 crucial traits of senescence, which are retarded, arrested, and in certain cases even reversed by SkQ1. The question arises why the SkQ-treated living creatures die in spite of obvious deceleration of the senescence program. The simplest explanation is that SkQ1 fails to arrest an age-linked decline of at least one physiological function of vital importance. Another possibility is that the aging program does not operate in very old organisms, who die due to accumulation of occasional injuries (as already mentioned, very old organisms are not interesting for evolution since they are too rare and their reproductive systems decline with age). To live much longer than usually, you need some “skill”, otherwise you may become a victim, e.g. of a simple but inevitable disproportion in construction of old organism, like in old pearl mussel (see above). Podospora anserina represents an interesting example in this context. This fungus seems to employ two scenarios for life, one short-lived (several weeks), and another very long-lived (years). Switching from the short- to long-lived modus vivendi can be achieved by growing P. anserina in a liquid medium [43]. A similar switch is observed also in P. anserina growing on a solid medium if mutations in respiratory chain or in mitochondrial morphology transition machinery take place [11, 12]. The effect of SkQ1 on the short-lived fungus was measurable but much smaller than the switch to the long-lived scenario. In fact, P. anserina responded to SkQ1 like three other species tested (C. affinis, D. melanogaster, and mice), i.e. by 1.5-2-fold increase in the median lifespan and by rectangularization of the survival curve. These relationships can be explained assuming that in SkQ1-treated P. anserina the execution of the aging program is inhibited, but SkQ does not induce a switch to the very long-lived modus vivendi.
Something similar was recently observed in the Dr. B. Cannon's group [44] who tested (within the framework of our project) the action of SkQ1 on progeric “mutator mice” with the D257A mutation in the proof-reading domain of mitochondrial DNA polymerase ã. Such a mutant polymerase can synthesize DNA but cannot recognize and correct its own errors in the process of this synthesis. As a result, frequency of mutations in mitochondrial DNA increases, entailing strong shortening in lifespan and premature development of numerous traits of senescence [45, 46]. It was found that SkQ1 prevents or retards appearance of such senescence traits as lordokyphosis, boldness, lowering of body temperature, torpor, cessation of estrual cycles, etc., and increases the lifespan but not as strongly as would be needed to return it to the normal level. Again, as in the above described wild-type mice, the SkQ1-receiving animals die without showing a senescence scenario. Preliminary results of histological analysis revealed that the most probable reason for death of the SkQ1-treated “mutator mice” is pathological changes in the colon epithelium [44], a disease specific for mice with mitochondrial mutations [47]. It should be noted that Cannon's experiments were done on the mutant animals living in a sterile vivarium. Generally, the effects of SkQ1 under these conditions resembled very much those on the wild-type mice living in the non-sterile vivarium used in the experiments described in this paper. The only difference is that infections were absent from reasons of death of “mutator mice”.
Concluding these and four preceding papers of the series [2-6], highly effective, mitochondria-targeted, rechargeable antioxidants composed of plastoquinone, decane linker, and a penetrating cation (SkQs) have been synthesized and tested on model membranes, isolated mitochondria, cell cultures, ex vivo organs, and living organisms. It is shown in experiments on fungus Podospora, invertebrates Ceriodaphnia and Drosophila, and mice that SkQ1 increases the lifespan at very much lower concentrations than previously known geroprotectors. This effect is accompanied by rectangularization of survival curve and (in mice) disappearance or retardation of many crucial traits of senescence. It is suggested SkQ1 is competent in switching off the aging (senescence) program responsible for a concerted decline of key physiological functions. Results obtained in experiments on this small molecule look promising for elaborating a drug prolonging youth.
We are very grateful to Professor V. A. Sadovnichii, Rector of Moscow State University, for his interest in the project and encouragement. We appreciate the help of N. M. Pleskatch and N. V. Smirnova in some experiments and technical assistance with electron microscopy of E. P. Senchenkov. We would like to express our sincere gratitude for valuable advice to Professor G. Blobel. Generous support of Mr. O. V. Deripaska, which in fact made possible this study, is greatly appreciated.
Supported by Mitotechnology LLC, M. V. Lomonosov Moscow State University, the Vol'noe Delo Foundation (grant No. 99F-06), Russian Ministry of Education and Science (grant “Leading Scientific Schools” No. 5762.2008.4).
REFERENCES
1.Skulachev, V. P. (2007) Biochemistry
(Moscow), 72, 1385-1396.
2.Antonenko, Y. N., Avetisyan, A. V., Bakeeva, L. E.,
Chernyak, B. V., Chertkov, V. A., Domnina, L. V., Ivanova, O. Yu.,
Izyumov, D. S., Khailova, L. S., Klishin, S. S., Korshunova, G. A.,
Lyamzaev, K. G., Muntyan, M. S., Nepryakhina, O. K., Pashkovskaya, A.
A., Pletjushkina, O. Yu., Pustovidko, A. V., Roginsky, V. A.,
Rokitskaya, T. I., Ruuge, E. K., Saprunova, V. B., Severina, I. I.,
Simonyan, R. A., Skulachev, I. V., Skulachev, M. V., Sumbatyan, N. V.,
Sviryaeva, I. V., Tashlitsky, V. N., Vassiliev, J. M., Vyssokikh, M.
Yu., Yaguzhinsky, L. S., Zamyatnin, A. A., Jr., and Skulachev, V. P.
(2008) Biochemistry (Moscow), 73, 1273-1287.
3.Bakeeva, L. E., Barskov, I. V., Egorov, M. V.,
Isaev, N. K., Kapelko, V. I., Kazachenko, A. V., Kirpatovsky, V. I.,
Kozlovsky, S. V., Lakomkin, V. L., Levina, S. V., Pisarenko, O. I.,
Plotnikov, E. Y., Saprunova, V. B., Serebryakova, L. I., Skulachev, M.
V., Stelmashook, E. V., Studneva, I. M., Tskitishvili, O. V.,
Vasilyeva, A. K., Victorov, I. V., Zorov, D. B., and Skulachev, V. P.
(2008) Biochemistry (Moscow), 73, 1288-1299.
4.Agapova, L. S., Chernyak, B. V., Domnina, L. V.,
Dugina, V. B., Efimenko, A. Yu., Fetisova, E. K., Ivanova, O. Yu.,
Kalinina, N. I., Khromova, N. V., Kopnin, B. P., Kopnin, P. B.,
Korotetskaya, M. V., Lichinitser, M. R., Lukashev, A. L., Pletjushkina,
O. Yu., Popova, E. N., Skulachev, M. V., Shagieva, G. S., Stepanova, E.
V., Titova, E. V., Tkachuk, V. A., Vasiliev, J. M., and Skulachev, V.
P. (2008) Biochemistry (Moscow), 73, 1300-1316.
5.Neroev, V. V., Archipova, M. M., Bakeeva, L. E.,
Fursova, A. Zh., Grigorian, E. N., Grishanova, A. Yu., Iomdina, E. N.,
Ivashchenko, Zh. N., Katargina, L. A., Khoroshilova-Maslova, I. P.,
Kilina, O. V., Kolosova, N. G., Kopenkin, E. P., Korshunov, S. S.,
Kovaleva, N. A., Novikova, Yu. P., Philippov, P. P., Pilipenko, D. I.,
Robustova, O. V., Saprunova, V. B., Senin, I. I., Skulachev, M. V.,
Sotnikova, L. F., Stefanova, N. A., Tikhomirova, N. K., Tsapenko, I.
B., Shchipanova, A. I., Zinovkin, R. A., and Skulachev, V. P. (2008)
Biochemistry (Moscow), 73, 1317-1328.
6.Esser, K. (1974) in Podospora anserina
(King, R. C., ed.) Plenum, New York, Vol. 1, pp. 531-551.
7.Borghouts, C., Kimpel, E., and Osiewacz, H. D.
(1997) Proc. Natl. Acad. Sci. USA, 94,
10768-10773.
8.Anisimov, V. N., Khavinson, V. Kh., Provinsiali,
M., Alimova, I. N., Baturin, D. A., Popovich, I. G., Zabezhinski, M.
A., Imyanitov, E. N., Mancini, R., and Franceschi, C. (2002) Int. J.
Cancer, 101, 7-10.
9.Kabak, Ya. M. (1968) Manual in Endocrinology
[in Russian], Vysshaya Shkola, Moscow.
10.Anisimov, V. N., Popovich, I. G., and
Zabezhinski, M. A. (2007) Meth. Mol. Biol., 371,
227-236.
11.Dufour, E., Boulay, J., Rincheval, V., and
Sainsard-Chanet, A. (2000) Proc. Natl. Acad. Sci. USA,
97, 4138-4143.
12.Scheckhuber, C. Q., Erjavec, N., Tinazli, A.,
Hammann, A., Nystrom, T., and Osiewacz, H. D. (2007) Nat. Cell
Biol., 9, 99-105.
13.Severina, I. I., et al., in preparation.
14.Skulachev, V. P. (1996) Quart. Rev.
Biophys., 29, 169-202.
15.Walker, D. W., and Benzer, S. (2004) Proc.
Natl. Acad. Sci. USA, 101, 10290-10295.
16.Berstein, L. (2004) Oncoendocrinology:
Traditions, Modernity and Prospects [in Russian], Nauka, St.
Petersburg.
17.Wei, M., Fabrizio, P., Hu, J., Ge, H., Cheng, C.,
Li, L., and Longo, V. D. (2008) PLoS Genetics, 4,
139-149.
18.James, A. M., Cocheme, H. M., and Murphy, M. P.
(2005) Mech. Ageing Dev., 126, 982-986.
19.Skulachev, V. P. (2005) IUBMB-Life,
57, 305-310.
20.Magwere, T., West, M., Riyahi, K., Murphy, M. P.,
Smith, R. A. J., and Partridge, L. (2006) Mech. Ageing Dev.,
127, 356-370.
21.Paul, A., Belton, A., Nag, S., Martin, I.,
Grotewiel, M. S., and Duttarov, A. (2007) Mech. Ageing
Dev., 128, 706-716.
22.Skulachev, V. P. (2005) Vestnik RAN,
75, 831-843.
23.Skulachev, V. P., and Longo, V. D. (2005) Ann.
N. Y. Acad. Sci., 1057, 145-164.
24.Mair, W., Goymer, P., Pletcher, S. D., and
Partridge, L. (2003) Science, 301, 1731-1733.
25.Libert, S., Zwiener, J., Chu, X., Vanvoorhies,
W., Roman, G., and Scott, D. (2007) Science, 315,
1133-1137.
26.Skulachev, V. P. (1999) Biochemistry
(Moscow), 64, 1418-1426.
27.Skulachev, V. P. (1999) Mol. Asp. Med.,
20, 139-184.
28.Skulachev, V. P. (2003) in Topics in Current
Genetics (Nyström, T., and Osiewacz, H. D., eds.) Model
Systems in Ageing, Springer-Verlag, Berlin-Heidelberg, Vol. 3, pp.
191-238.
29.Goldsmith, T. C. (2008) J. Theor. Biol.,
252, 764-768.
30.Lambert, A. J., Boysen, H. M., Buckingham, J. A.,
Yang, T., Podlutsky, A., Austad, S. N., Kunz, T. H., Buffenstein, R.,
and Brand, M. D. (2007) Aging Cell, 6, 607-618.
31.Buffenstein, R. (2005) J. Gerontol. Biol.
Sci., 60, 1369-1377.
32.Andziak, B., and Buffenstein, R. (2006) Aging
Cell, 5, 525-532.
33.Andziak, B., O'Connor, T. P., Qi, W., DeWaal, E.
M., Pierce, A., Chaudhuri, A. R., van Remmen, H., and Buffenstein, R.
(2006) Aging Cell, 5, 463-471.
34.Andziak, B., O'Connor, T. P., and Buffenstein, R.
(2005) Mech. Ageing Dev., 126, 1206-1212.
35.Labinsky, N., Csiszar, A., Orosz, Z., Smith, K.,
Rivera, A., Buffenstein, R., and Ungvari, Z. (2006) Am. J. Physiol.
Heart Circ. Physiol., 291, H2698-H2704.
36.Migliaccio, E., Giorgio, M., Mele, S., Pelicci,
G., Revoldi, P., Pandolfi, P. P., Lanfrancone, L., and Pelicci, P. G.
(1999) Nature, 402, 309-313.
37.Liu, X., Jiang, N., Hughes, B., Bigras, E.,
Shoubridge, E., and Hekimi, S. (2006) Gen. Dev., 19,
2424-2434.
38.Chu, H.-P., Merkin, J. J., Grigorian, I. A.,
Dorovkov, M. V., Clifford, P., Nagele, R. G., Komarova, E. A., Gudkov,
A. V., Yen, K., Mobbs, D. E., Harrison, D. E., and Ryazanov, A. G.
(2008) (submitted).
39.Park, T. J., Lu, Y., Juttner, R., Smith, E. St.
J., Hu, J., Brand, A., Wetzel, C., Milenkovic, N., Erdmann, B.,
Heppernstall, P. A., Laurito, C. E., Wilson, S. P., and Lewin, G. R.
(2008) PLoS Biology, 6, 156-170.
40.Pawelec, G., and Larbi, A. (2008) Exp.
Gerontol., 43, 34-38.
41.Saule, P., Trauet, J., Dutriez, V., Lekeux, V.,
Dessaint, J. P., and Labalette, M. (2005) Mech. Ageing Dev.,
127, 274-281.
42.Polyakova, V. O., Kvetnoy, I. M., Khavinson, V.
Kh., Maryanovich, A. T., and Konovalov, S. S. (2001) Adv.
Gerontol., 8, 50-57.
43.Turker, M. S., and Cummings, D. J. (1987) J.
Bacteriol., 169, 454-460.
44.Shabalina, I. G., et al. (in preparation).
45.Trifunovic, A., Wreeenberg, A., Falkenberg, M.,
Spelbrink, J. N., Rovio, A. T., Bruder, C. E., Bohlooly, Y. M., Gidlof,
S., Oldfors, A., Wilbom, R., Tornell, J., Jacobs, H. T., and Larsson,
N.-G. (2004) Nature, 429, 417-423.
46.Kujoth, G. C., Hiona, A., Pugh, T. D., Someya,
S., Panzer, K., Wohlgemuth, S. E., Hofer, T., Seo, A. Y., Sullivan, R.,
Jobling, W. A., Morrow, J. D., van Remmen, H., Sedivy, J. M., Yamasoba,
T., Tanokura, M., Weindruch, R., Leeuwenburgh, C., and Prolla, T. A.
(2005) Science, 309, 481-484.
47.Greaves, L. C., Preston, S. L., Tadrous, P. J.,
Taylor, R. W., Barron, M. J., Oukrif, D., Leedham, S. J., Deheragoda,
M., Sasieni, P., Novelli, M. R., Jankowski, J. A., Turnbull, D. M.,
Wright, N. A., and McDonald, S. A. (2006) Proc. Natl. Acad. Sci.
USA, 103, 714-719.