Structure and Characterization of the Gene Cluster of the O-Antigen of Escherichia coli O49 Containing 4,6-Dideoxy-4-[(S)-3-hydroxybutanoylamino]-D-glucose
A. V. Perepelov1*, Quan Wang2,3, S. N. Senchenkova1, S. D. Shevelev1, A. S. Shashkov1, Lu Feng2,3, Y. A. Knirel1, and Lei Wang2,3
1Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky pr. 47, 119991 Moscow, Russia; fax: (495) 137-6148; E-mail: perepel@ioc.ac.ru2TEDA School of Biological Sciences and Biotechnology and 3Tianjin Key Laboratory for Microbial Functional Genomics, TEDA College, Nankai University, 23 HongDa Street, TEDA, Tianjin 300457, P. R. China; E-mail: wangquan@mail.nankai.edu.cn
* To whom correspondence should be addressed.
Received July 11, 2007
An O-polysaccharide was isolated by mild acid degradation of the lipopolysaccharide of enteropathogenic Escherichia coli O49 and studied by sugar analysis along with one- and two-dimensional 1H- and 13C-NMR spectroscopy. The following structure of the linear tetrasaccharide repeating unit of the O-polysaccharide was established:where d-Qui4N(S3HOBut) stands for 4,6-dideoxy-4-[(S)-3-hydroxybutanoylamino]-d-glucose and O-acetylation of GlcNAc is partial (~30%). To our knowledge, no N-(3-hydroxybutanoyl) derivative of Qui4N has been hitherto found in bacterial polysaccharides. Gene functions of the O-antigen gene cluster of E. coli O49 were assigned by bioinformatics analysis and found to correspond to the O-polysaccharide structure. Two new genes were revealed and suggested to be responsible for synthesis and transfer of the 3-hydroxybutanoyl group.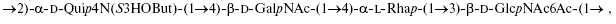
KEY WORDS: Escherichia coli, lipopolysaccharide, bacterial polysaccharide structure, 3-hydroxybutyrate, 4-amino-4,6-dideoxy-d-glucose, O-antigen gene clusterDOI: 10.1134/S0006297908040044