Oxidative Stress and Formation and Maintenance of Root Stem Cells
V. B. Ivanov
Timiryazev Institute of Plant Physiology, Russian Academy of Sciences, Botanicheskaya ul. 35, 127276 Moscow, Russia; fax: (495) 970-8018; E-mail: ivanov@ippras.ru
Received May 24, 2007
The hypothesis of L. Feldman and his coworkers, according to which a more oxidizing environment in the cells of root quiescent center results from high activity of ascorbate oxidase activated by indoleacetic acid (IAA) accumulating in these cells, is discussed. The high activity of ascorbate oxidase is responsible for lowered concentrations of the reduced form of ascorbic acid and glutathione and high content of reactive oxygen species in quiescent center cells. The oxidative stress represses proliferation of the cells. Inhibitors of IAA transport attenuate the oxidative stress, thus suggesting a role of IAA as an activator of ascorbate oxidase. Interestingly, the high concentration of IAA in dividing cap cells adjacent to the quiescent center cells did not cause retardation of cell proliferation and oxidative state in these cells.
Key words: oxidative stress, stem cells, quiescent center, meristem, root, ascorbate oxidase, IAADOI: 10.134/S0006297907100082
Abbreviations: IAA) indoleacetic acid; NPA) naphthyl phthalic acid; ROS) reactive oxygen species.
One of the most intensively developing fields of current biology is
investigation of stem cells. Plant stem cells are of great interest
because of their particular features. Unlike animal stem cells, the
plant stem, actively proliferating, and differentiated plant cells
repeatedly alternate between these states. This allows plants, unlike
most vertebrates, to grow and form new organs throughout their life and
provides their ability for vegetative propagation. The ability of
plants to form new organs throughout their life and to propagate
results, on one hand, from a prolonged maintenance of meristems
containing undifferentiated cells capable of dividing and, on the other
hand, from the ability of differentiated plant cells to resume
divisions and to form new organs and even whole plants. Although the
meristematic cells can potentially divide for a long time, most cells
divide only several times before they escape meristem and are
substituted by new cells arising from apical cells. Only the most
apical cells remain in meristems for a long time and, unlike most
meristematic cells, divide very rarely and remain quiescent. Their
protein and RNA synthesis rates are very low, concentration of these
substances is decreased, and features of quiescent cells are evident
(they are slightly basophilic, nucleoli are small, chromatin is of
typical structure, etc.). In roots, these cells were called the
“quiescent center” by F. Clowes [1]. The
cells of quiescent center can be clearly revealed on autoradiograph of
roots after prolonged incubation in [H3]thymidine solution
or on sections stained with basic or acidic dyes. Unlike superior
cells, the duration of their mitotic cycles is 6-10 times longer,
mostly due to elongation of G1-phase [2]. Apical meristems of stem contain similar cells
named the “d'attente meristem”. They divide rarely during
vegetative growth, but start active divisions upon initiation of
reproductive organs.
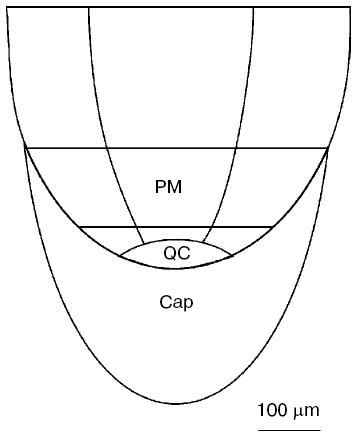
In the present paper, we shall confine ourselves to works studying the root quiescent center, which are regarded in a number of reviews [3-8]. The quiescent center is present in all plants, except roots with short growth period, for instance, specialized short roots in the root system of milkweed [9] or roots of cacti, which elongate only several days after germination [10]. The size of the quiescent center depends on the root thickness [11, 12]. In thick roots of horse beans or maize, it consists of several hundreds of cells (Fig. 1), whereas in thin roots, for instance, in Arabidopsis, it consists of four cells only.
When lateral roots are produced, the quiescent center is formed after the appearance of the lateral root from the main root. The number of lateral roots formed on the main root is many times higher than the number of cells comprising the quiescent center. Moreover, cells are growing as a symplast in plants, and no cell transfer happens. Therefore, the formation of quiescent center when a lateral root is developing occurs via transition into quiescent state of the most apical cells of the developing spire of lateral root adjacent directly to the root cap. These transitions of actively proliferating cells to dormant state happen repeatedly during morphogenesis. They are also observed during root regeneration after decapitation. When cutting a part of the root tip not exceeding critical size determined by the root thickness, the root is capable of forming a new root cap and quiescent center, and the same root can be decapitated repeatedly [13, 14]. A new quiescent center appears from actively proliferating cells upon regeneration.Fig. 1. Scheme of apical cone of maize root. PM, proximal meristem; QC, quiescent center [31].
Quiescent center cells have diverse functions. First, the formation of quiescent center, whose cells are dividing 6-10 times less frequently than other meristematic cells, results in drastic decrease in the maximum number of mitoses during the entire growth time period. So, for instance, 100-120 mitoses must be completed in maize root to achieve 2.0-2.5 m in length. However, since the first cells divide very rarely, but their progeny replaces the rest of meristem cells as time goes on, they divide 15-17 times [3]. Obviously, chromosomal aberrations arising in the main meristem part expand to a limited number of cells, whereas aberrations arising in the first cells can be found in all cells with time. Since the first cells have 6-10 times longer mitotic cycles, mostly due to elongation of G1 phase, they are much more resistant, and thereby the root decreases the possibility of spreading of chromosomal aberrations. The high resistance of cells in quiescent center to unfavorable influences enables the root to resume its growth after damage. After irradiation with doses insufficient for complete inhibition of divisions, mitoses become arrested in the main part of meristem, and cell divisions of the quiescent center become activated. This results in growth restoration after the cells formed from the quiescent center cells pass on to elongation [15]. A similar phenomenon is observed after incubation of roots of thermophilic plants under low temperature above zero. In this case, meristematic cells become damaged due to chromosomal adhesion, and their proliferation is disturbed, whereas the quiescent center cells begin to rapidly divide after transfer of the plants to normal conditions [16, 17]. Second, the quiescent center provides the storage of relatively slightly differentiated cells, which are capable of changing the direction of their differentiation comparatively easily under particular conditions. This facilitates formation of a new root cap after removal or damage of the previous root cap, which plays an important role in root growth providing root defense, participating in geotropic response, and possibly in other tropisms. After root cap removal or damage, the quiescent center cells begin dividing very rapidly to form a new root cap. Although the root cap can be made of other cells, its restoration from the quiescent center is the most simple and rapid process. Third, according to the opinion of some investigators [18-20], the quiescent center inhibits differentiation of adjacent cells and maintains them in the stem cell proliferating state. After one of the quiescent center cells in Arabidopsis root was burned with a laser, an adjacent root cap columella cell arrests its division and deposits starch. The stimulation of the cell to the starch formation, which indicates the transition to differentiation, is not associated directly with cessation of divisions [18]. Besides, in mutants in genes essential for the formation of quiescent center, the root growth stops soon after its germination, and cells cannot divide for a long time [20, 21].
The quiescent center cells are reminiscent of animal stem cells in their features, and some authors regard them as plant stem cells. The quiescent center cells have no tissue markers which can be revealed in cells localized above them, they can proliferate, they are maintained for all the period of root growth, their derivatives can form all root cells, and meristem is restored from them after its damage [3, 5, 8]. However, according to the opinion of other investigators [18-20] the quiescent center is an organizing center of root, with the stem cells surrounding it, because the quiescent center cells divide extremely rarely and, according to the opinion of several investigators, they influence the behavior of adjacent cells. A comparison of these points of view has been made [7].
The mechanisms of formation and maintenance of the quiescent center are not yet clear. It should be first noted that the maintenance of the quiescent center in a root is an active process. After isolated roots growing in sterile culture were transferred on medium without sucrose, the quiescent center cells began to divide on the addition of sucrose [22]. We also observed (unpublished data) the division of quiescent center cells after maize rootlets were cut out and transferred into a humid environment on a filter paper wetted with water.
It has been demonstrated using molecular genetics methods that the formation and maintenance of quiescent center is determined by several genes responsible for the formation of indoleacetic acid (IAA) transporter proteins [24] and transcription factors [19-21, 25]. However, the functional role of proteins, whose synthesis is essential, still remains unclear.
The formation and maintenance of quiescent center depends on IAA translocated to meristem top-down; this is proved by a number of facts: i) high concentration of IAA in the quiescent center cells demonstrated by immunohistochemical methods [26] and expression of early IAA response genes shown by using GUS [23]; ii) transition of quiescent center cells to active divisions and meristem opening under the inhibition of IAA transport with triiodobenzoic [26] or N-naphthyl phthalic (NPA) acids, as well as disturbance of root regeneration by IAA after decapitation [23, 27, 28]; iii) the lack of quiescent center in mutant roots with impaired transport of IAA [29]; iv) altered localization of gene expression specific for quiescent center under inhibition of IAA transport [23]; v) IAA-dependence of expression of PLT1 and PLT2 genes essential for maintenance of quiescent center cells [30]. Also, the presence of root cap plays an important role in the formation of quiescent center [8].
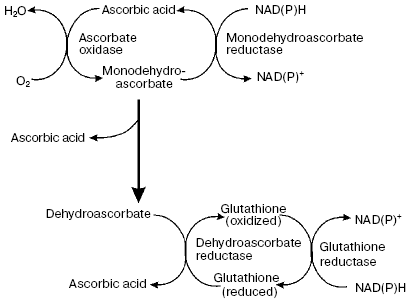
Another putative mechanism of IAA action on quiescent center cells--stimulation of ascorbate oxidase activity leading to the development of typical features of oxidative stress--is of great interest. This hypothesis was developed in works of L. Feldman with coworkers [8, 26, 27, 31, 32] who studied maize roots. They used a maize variety in which quiescent center cells can be relatively easily isolated with a cataract scalpel because of their thick cell walls. This makes it possible to determine the content of several compounds and various enzymatic activities in quiescent center cells and compare these with the data on above localized cells of root proximal meristem (Fig. 1). It has been demonstrated that the content of reduced ascorbate and glutathione in quiescent center cells is substantially decreased in comparison with cells located immediately above the proximal meristem, whereas the levels of oxidized forms of these compounds are substantially higher (Tables 1 and 2). This is associated with elevated activity of ascorbate oxidase in quiescent center cells and reduction of glutathione reductase activity. The activities of ascorbate oxidase and glutathione reductase in quiescent center cells were 3.7 and 8.6 times lower, respectively, than in proximal meristem cells. High ascorbate oxidase activity in quiescent center cells was demonstrated by histochemical methods [33]. IAA is known to stimulate ascorbate oxidase activity. The elevation of its activity leads to oxidation of ascorbate, which in turn results in glutathione oxidation via the ascorbate/glutathione cycle (Fig. 2). Activation of the quiescent center cell divisions occurs, and it cannot be revealed on root sections as an area of cells not synthesizing DNA when the roots are incubated in ascorbate solution [34].
Table 1. Ascorbate content in proximal
meristem and quiescent center of maize roots (control, 48-h root; NPA,
root treated with N-naphthyl phthalic acid (10-5 M) for
24 h (from [31]))
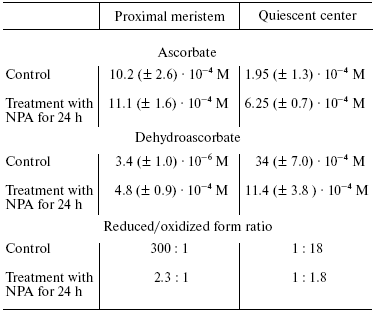
Table 2. Content of reduced (GSH) and
oxidized (GSSG) glutathione in proximal meristem and quiescent center
of maize root (control, 48-h root; NPA, rootlets treated with
N-naphthyl phthalic acid solution (10-5 M) for
24 h (from [31]))
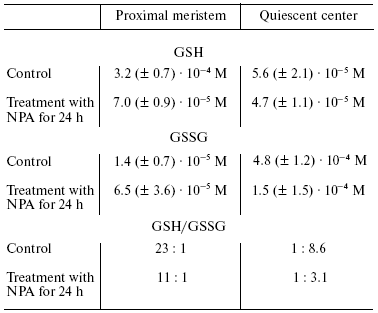
The role of IAA is proved by substantial change in the content of oxidized compounds under the action of N-naphthyl phthalic acid, an effective selective inhibitor of IAA transport. So, the development of oxidative stress conditions occurring in quiescent center cells is associated with the elevation of IAA concentration. This results in 15.6- and 34-fold increase in O2-* and H2O2 contents, respectively, in its cells in comparison with the cells of proximal meristem [31]. More oxidized conditions in quiescent center cells can be revealed using a fluorescent probe to reactive oxygen species (ROS)--carboxy-H2DCFDA--whose fluorescence is revealed in quiescent center cells, but not in proximal meristem surrounding them [32]. In the same work it has been demonstrated by using other potential-dependent fluorescent probes JC-9 and Mitotracker Orange, that mitochondria of quiescent center cells have lower potential in comparison with meristem cells, indicating depolarization of inner mitochondrial membrane of quiescent center cells and their decreased ability to synthesize ATP. Activity of Krebs cycle enzymes was drastically decreased in quiescent center cells in comparison with proximal meristem cells, and pyruvate dehydrogenase activity was not detected at all. Activity of nucleus-encoded transcripts of genes for mitochondrial proteins was also significantly decreased in quiescent center cells [32].Fig. 2. Ascorbate-glutathione cycle (from [8]).
Thus, the data demonstrate the possibility of development of oxidative stress in quiescent center as a result of IAA accumulation in its cells. This makes probable a suggestion that stimulation of oxidative processes leading to the decrease of reduced ascorbate and glutathione contents, increase in ROS, i.e. formation of oxidative stress conditions, is one of the mechanisms by means of which the important plant phytohormone IAA influences the formation and maintenance of quiescent center. There are numerous data suggesting that proliferation of plant and animal cells slows down under oxidative stress (see for example [8, 35-37]). Interestingly, initial cells of root cap subjacent to quiescent center also contain high concentration of IAA, but these cells divide more often than other meristem cells and do not experience oxidative stress. Causes of these differences are not discussed in the literature. Mechanisms of formation and maintenance of quiescent center are apparently more complex, but this does not mean that further studies of oxidative stress in the quiescent center cells are not important. Should the mechanism of IAA influence on the development of oxidative stress and formation of quiescent center in the root proposed by Feldman and coworkers be the case, then we would have an interesting example of dependence of important morphogenetic processes on redox state of cells.
The author is indebted to Academician V. P. Skulachev for discussion of problems regarded in this paper.
This work was partially supported by the Russian Foundation for Basic Research (grant No. 06-04-48861).
REFERENCES
1.Clowes, F. A. L. (1956) New Phytologist,
55, 29-35.
2.Clowes, F. A. L. (1963) Brookhaven Symp.
Biol., 16, 46-58.
3.Ivanov, V. B. (1974) Cellular Basics of Plant
Growth [in Russian], Nauka, Moscow.
4.Clowes, F. A. L. (1975) The Development and
Function of Plants (Torrey, J. G., and Clarkson, D. T., eds.)
Academic Press, London, pp. 3-19.
5.Barlow, P. W. (1978) Stem Cells and Tissue
Homeostasis (Lorel, B. I., Potten, C. S., and Cole, R. J., eds.)
Cambridge University Press, Cambridge, pp. 87-113.
6.Ivanov, V. B. (2003) Rus. J. Dev. Biol.,
34, 205-212.
7.Ivanov, V. B. (2004) Rus. J. Plant
Physiol., 51, 834-847.
8.Jiang, K., and Feldman, L. J. (2005) Annu. Rev.
Cell Dev. Biol., 21, 485-509.
9.Raju, M. V. S., Steeves, T. A., and Naylor, J. M.
(1964) Can. J. Bot., 42, 1615-1628.
10.Rodriguez-Rodriguez, J. F., Shishkova, S.,
Napsucialy-Mendivil, S., and Dubrovsky, J. (2003) Planta,
217, 849-857.
11.Clowes, F. A. L. (1984) New Phytol.,
96, 13-21.
12.Barlow, P. W., and Rathfelder, E. L. (1984)
Ann. Bot., 53, 249-260.
13.Feldman, L. J. (1976) Planta, 128,
207-212.
14.Ivanov, V. B., and Larina, L. P. (1983) Dokl.
Akad. Nauk SSSR, 272, 1014-1017.
15.Clowes, F. A. L. (1963) Brookhaven Symp.
Biol., 16, 46-58.
16.Clowes, F. A. L., and Stewart, H. E. (1967)
New Phytologist, 66, 115-125.
17.Barlow, P. W., and Rathfelder, E. L. (1985)
Environ. Exp. Bot., 25, 303-314.
18.Van den Berg, C., Willemsen, V., Hage, W.,
Weisbeek, P., and Scheres, B. (1995) Nature, 378,
62-65.
19.Scheres, B. (2007) Nat. Rev. Mol. Cell
Biol., 8, 345-354.
20.Sarkar, A. R., Luijten, M., Miyashima, S.,
Lenhard, M., Hashimoto, T., Narajima, K., Scheres, B., Heidstra, R.,
and Laux, T. (2007) Nature, 446, 811-814.
21.Sabatini, S., Heidstra, R., Wildwater, M., and
Scheres, B. (2003) Gen. Dev., 17, 354-358.
22.Webster, P. L., and Langenhauer, H. D. (1973)
Planta, 112, 91-110.
23.Sabatini, S., Beis, D., Wolkenfelt, H., Murfelt,
H., Guilfole, T., Malamy, J., Benfey, P., Leyser, O., Weisbeek, P., and
Scheres, B. (1999) Cell, 99, 463-472.
24.Billou, I., Xu, J., Wildwater, M., Willemsen, V.,
Paponov, I., Friml, J., Heidstra, R., Aida, M., Palme, K., and Scheres,
B. (2005) Nature, 433, 39-44.
25.Wildwater, M., Campiho, A., Perez-Perez, J. M.,
Heidstra, R., Blilou, I., Korthout, H., Chatterjee, J., Mariconti, L.,
Gruissem, W., and Sheres, B. (2005) Cell, 123,
1337-1349.
26.Kerk, N., and Feldman, L. (1995)
Development, 121, 2825-2833.
27.Jiang, K., and Feldman, L. J. (2003) J. Plant
Growth Regul., 21, 432-440.
28.Ponce, G., Lujan, R., Campos, M. E., Reyes, A.,
Nieto-Satelo, J., Feldman, L., and Cassab, G. I. (2000) Planta,
211, 23-33.
29.Friml, J., Benkova, E., Blilou, I., Wisniewska,
J., Hamann, T., Ljung, K., Woody, S., Sandberg, G., Scheres, B.,
Jurgens, G., and Palme, K. (2002) Cell, 108, 661-673.
30.Aida, M., Vernoux, T., and Furutani, M. (2002)
Development, 129, 3965-3974.
31.Jiang, K., Meng, Y. L., and Feldman, L. J. (2003)
Development, 130, 1429-1438.
32.Jiang, K., Ballinger, T., Li, D., Zhang, S., and
Feldman, L. (2006) Plant Physiol., 140, 1118-1125.
33.Liso, R., De Tullio, M. C., Ciraci, S.,
Balestrini, R., La Rocca, N., Bruno, L., Chiappetta, A., Bitoni, M. B.,
Bonfante, P., and Arrigoni, O. (2004) J. Exp. Bot., 55,
2589-2597.
34.Liso, R., Innocenti, A. M., Bitonti, B. M., and
Arrigoni, O. (1988) New Phytol., 110, 469-471.
35.Smith, J., Ladi, E., Mayer-Proschel, M., and
Noble, M. (2000) Proc. Natl. Acad. Sci. USA, 97,
10032-10037.
36.Boonstra, J., and Post, J. M. (2004) Gene,
337, 1-13.
37.Menon, S. G., Sarsour, E. H., Spitz, D. R.,
Higashikubo, R., Sturm, M., Zhang, H., and Goswami, P. C. (2003)
Cancer Res., 63, 2109-2117.