Biogenesis of Chlorophyll-Binding Proteins under Iron Stress in Synechocystis sp. PCC 6803
X. Liu, J. Zhao, and Q. Wu*
Department of Biological Sciences and Biotechnology, Tsinghua University, Beijing 100084, P. R. China; fax: 86-10-62781825; E-mail: qingyu@tsinghua.edu.cn* To whom correspondence should be addressed.
Received June 3, 2005; Revision received June 27, 2005
The biogenesis of chlorophyll-binding proteins under iron stress has been investigated in vivo in a chlN deletion mutant of Synechocystis sp. PCC 6803. The chlN gene encodes one subunit of the light-independent protochlorophyllide reductase. The mutant is unable to synthesis chlorophyll in darkness, causing chlorophyll biosynthesis to become light dependent. When the mutant was propagated in darkness, essentially no chlorophyll and photosystems were detected. Upon return of the chlN deletion mutant to light, 77 K fluorescence emission spectra and oxygen evolution of greening cells under iron-sufficient or -deficient conditions were measured. The gradual blue shift of the photosystem I (PS I) peak upon greening under iron stress suggested the structural alteration of newly synthesized PS I. Furthermore, the rate of biogenesis of PS II was delayed under iron stress, which might be due to the presence of IsiA.
KEY WORDS: chlorophyll synthesis, chlorophyll-binding proteins, cyanobacterium, fluorescence emission, iron stress, IsiADOI: 10.1134/S0006297906130177
Abbreviations: Chl) chlorophyll; LAHG) light activated heterotrophic growth; PC) phycocyanin; PS I) photosystem I; PS II) photosystem II.
Cyanobacteria, like many other prokaryotes, are often exposed to
iron-deficient environments due to the extremely low solubility of the
dominating Fe3+ ions under oxygenic conditions. Iron
deficiency results in a variety of physiological and morphological
changes in cyanobacteria. The overall changes are as follows: decrease
in phycocyanins (PC) and chlorophyll (Chl) content, a characteristic
blue shift of the main red maximum of Chl absorption, dramatic
alterations in the 77 K Chl fluorescence emission spectrum, reduction
in the number of thylakoids, reduced photosystem II (PS II) and
photosystem I (PS I) photochemistry, and partial replacement of
ferredoxin by flavodoxin [1-3].
Most importantly, a novel Chl-binding protein, IsiA, encoded by
isiA, is synthesized. In iron-deficient cyanobacteria up to 50%
of Chl is associated with it [4]. Because of the
similarity to CP43, IsiA was suggested to be associated with PS II
functioning as a Chl storage protein [5] or an
excitation energy dissipater [6, 7]. Recently, it was shown that 18 copies of IsiA form
a ring around the trimeric PS I reaction center. This would imply a
substantial increase of the PS I antenna size [8,
9]. Recent studies utilizing biochemical, physical,
and genetic approaches have revealed the structural and functional
rearrangement of the photosynthetic apparatus under iron stress (for a
review, see [10]). In contrast, little is known
about the biogenesis of these Chl-binding proteins. Understanding of
the contribution of Chl molecules to functional photosynthetic
complexes assembly under iron stress remains especially elusive.
Synechocystis sp. PCC 6803 contains both light-dependent and light-independent Chl biosynthesis pathways [11]. Three genes (chlL, chlN, and chlB) encode the subunits of the light-independent protochlorophyllide reductase. Mutants lacking any one of these genes are unable to synthesize Chl in darkness, causing Chl synthesis to become light dependent [12, 13]. When they were propagated under light activated heterotrophic growth conditions (LAHG, in darkness except for 15 min/day of weak light), the mutant cells become etiolated and contain essentially no Chl and photosystems [12, 14]. In chlN- mutant, Chl content is controlled by light, and the process of “greening” (light-dependent Chl synthesis and photosystem assembly) can be monitored. Therefore, this mutant strain provides an excellent in vivo system to investigate biogenesis of Chl-binding proteins in relation to Chl biosynthesis.
In this work, this system was used to study the biogenesis of Chl-binding proteins under iron stress. We have performed both spectral and functional analyses under in vivo conditions to study the biogenesis of the photosynthetic apparatus in relation to Chl biosynthesis under iron deficiency.
MATERIALS AND METHODS
Culture and growth. Both wild-type and mutant strains of Synechocystis sp. PCC 6803 were cultured at 30°C (±1) in BG 11 medium [15] containing either 30 µM ferric ammonium citrate or lacking this component completely. Cells were grown in continuous light (white light at 50 µE*m-2*sec-1) or under LAHG conditions. Under LAHG conditions, the cells were kept in complete darkness except for one 15-min light period (white light at 50 µE*m-2*sec-1) every 24 h. Aeration was provided through bubbling. The cells were harvested during the late logarithmic growth phase (around 8*107 cells/ml) by centrifugation at 6000g for 7 min and resuspended in fresh medium. Cell growth was determined by monitoring the optical density of the culture at 730 nm using an Ultra-Spec 2000 UV/Visible spectrophotometer (Pharmacia, Sweden). The specific growth rate was defined as an increase in OD730 during the time indicated.
Chlorophyll analysis. Chlorophyll was extracted with 100% methanol from cell pellets obtained from 1 ml of Synechocystis sp. PCC 6803 wild-type and mutant cell cultures (OD730 = 0.8) that had been grown in light or under LAHG conditions. The Chl content was determined according to MacKinney [16].
Measurements of PC contents, absorption spectra, and 77 K fluorescence emission spectra. Absorption spectra of intact Synechocystis sp. PCC 6803 cells were measured by an Ultra-Spec 2000 UV/Visible spectrophotometer and the relative PC amount was determined according to Xu et al. [17]. Low temperature (77 K) fluorescence measurements were performed on a F4500 spectrofluorimeter (Hitachi, Japan). The cells were suspended in 25 mM Hepes-NaOH, pH 7.0. Cell suspensions were adjusted to equal Chl concentration within the range of 2-10 µg Chl/ml. Cells were immediately frozen in liquid nitrogen without any further pretreatment.
Measurement of oxygen evolution. Oxygen evolution was measured with a Clark-type electrode (Hansatech, England) at 25°C. Cells were suspended in 25 mM Hepes-NaOH, pH 7.0. Light was provided by a 150-W xenon arc lamp. The incident light intensity was 1500 µE*m-2*sec-1.
RESULTS AND DISCUSSION
Growth and pigment composition. The chlN- mutant cells exhibit similar growth rates compared to wild-type cells under iron-sufficient and -deficient conditions although the cell growth rates in darkness are lower than those in light (Table 1). These results indicate that iron stress and deletion of chlN do not affect the growth and viability of the cells. After growth under LAHG conditions for two weeks, the Chl content in the mutant cells was about 5-7% of that in the wild-type in both iron-sufficient and -deficient media. In addition, the Chl content decreased under iron stress in both wide-type and mutant cells compared to the control (Table 1).
Table 1. Characteristics of
Synechocystis sp. PCC 6803 wild-type and the
chlN- mutant cells grown in iron-sufficient (+) and
-deficient (-) media (cells were grown in continuous light or under
LAHG conditions for two weeks; mean ± S.D., n =
3)
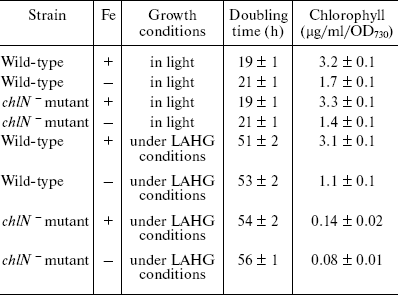
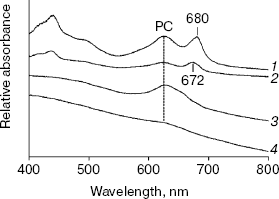
Figure 1 shows the absorption spectra of intact cells of chlN- mutant grown under iron-sufficient and -deficient conditions. The spectrum of cells in light has three major peaks: the peaks at 436 and 680 nm correspond to Chl [18], whereas that at 625 nm is due to PC, the major component of the phycobilisome [19]. As expected, the spectrum of chlN- cells in darkness lacked the two Chl absorption peaks and retained the PC absorption peak. The PC absorption peak under iron stress decreased significantly both in light and darkness. Indeed, the PC level under iron deficiency was 15-20% of that under iron sufficiency, regardless of the Chl content (data not shown).
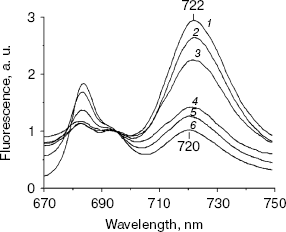
77 K Chl fluorescence emission under iron stress. The Chl fluorescence emission spectra of iron-sufficient wild-type cells at 77 K exhibit characteristic peaks centered at 684 nm (Chl antenna of PS II), 695 nm (CP47 of PS II), and 722 nm (PS I Chl-protein complex) [20]. Transferring the iron-sufficient cells to an iron-deficient medium caused a gradual increase in the 684 nm peak and a decrease in the PS I associated peak at 722 nm. Concomitant with this, a gradual shift in the PS I related peak from 722 to 720 nm was registered. These gradual changes completed after 72 h, resulting in a dramatic change in the emission spectrum (Fig. 2). The specific increase in the 685 nm peak and a typical blue shift in the red Chl absorption peak (Fig. 1) are characteristic of the induction of IsiA expression [8]. The blue shift in the PS I characteristic peak was also observed in Synechococcus 7942, which is suggested to be a structural alteration in the PS I centers during acclimation to iron deficiency [3]. This shift is not a simple consequence of the IsiA-PS I supercomplex, because it was not observed in a mutant overproducing IsiA [9].Fig. 1. Absorption spectra of chlN- cells grown in iron-sufficient (1, 3) and -deficient (2, 4) media. The spectrum of the chlN- mutant was determined after growth in continuous light (1, 2) or under LAHG conditions (3, 4) for two weeks.
No differences in 77 K Chl fluorescence emission spectra were observed between the wild-type and chlN- strains grown in light. However, in chlN- mutant grown under LAHG conditions, no significant fluorescence emission that correlated with PS II or PS I could be detected under iron-sufficient and -deficient conditions (data not shown).Fig. 2. Time course of 77 K Chl fluorescence emission spectra of wild-type cells after a shift from iron-sufficient to -deficient conditions. Spectra were taken after cells had been transferred from iron-sufficient to -deficient conditions for 0 (1), 6 (2), 12 (3), 24 (4), 48 (5), and 72 h (6). The excitation wavelength was 435 nm. The spectra are normalized to 1.0 at 695 nm.
Biogenesis of photosynthetic complexes in relation to Chl biosynthesis under iron stress. By deletion of chlN, a system in which Chl synthesis can be triggered by light was set up. By means of this system, it is possible to investigate the biogenesis of the Chl-binding proteins in relation to Chl biosynthesis under iron stress. To address this question, both diagnostic (fluorescence emission spectra) and functional (oxygen evolution) analyses were performed.
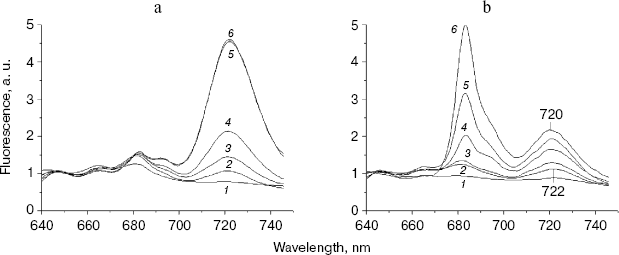
First of all, 77 K fluorescence emission spectra were measured using the chlN- cells under iron-sufficient and -deficient conditions at their different stages of greening (Fig. 3). Under the iron-sufficient condition, a 684 nm fluorescence emission peak became apparent during the early stages of greening and was maximal after about 12 h of greening. The 722 nm emission could be detected after 6 h of greening and increased until about 48 h after the initiation of Chl synthesis. The 695 nm emission band appeared only after 24 h of illumination. Finally, a normal pattern of the fluorescence emission spectrum similar to that of the wild-type grown in light was established at 48 h of greening (Fig. 3a). The results in Fig. 3b show the biogenesis of photosystems under iron-deficient conditions. After 6 and 12 h of greening, no difference was observed between the iron-deficient and -sufficient cells. The 695 nm peak was also observed only after 24 h of greening as in iron-sufficient cells. However, the 684 nm peak increased sharply after 24 h of illumination until about 72 h after the initiation of Chl synthesis. In addition, the 722 nm emission was blue shifted to 720 nm gradually until about 72 h of greening.
At the time cells were transferred to the light, both the chlN- cells under iron-sufficient and -deficient conditions exhibited no oxygen evolution. Correlating with fluorescence measurements, the oxygen evolution rates increased to about the maximal rates after 48 h of greening under iron-sufficient conditions and after 72 h of greening under iron-deficient condition (Table 2). This indicates that the rate of biogenesis of functional PS II is delayed under iron stress.Fig. 3. Chlorophyll fluorescence emission spectra of chlN- cells measured at 77 K at different stages of greening under iron-sufficient (a) and -deficient (b) conditions. The time between the exposure of the chlN mutant grown under LAHG conditions to continuous light and measuring the spectra was 0 (1), 6 (2), 12 (3), 24 (4), 48 (5), and 72 h (6). The excitation wavelength was 435 nm. The spectra are normalized to 1.0 at 650 nm.
Table 2. Rates of oxygen evolution in
greening cells grown in iron-sufficient (+) and -deficient (-) media
(all cells were propagated under LAHG conditions for two weeks and then
transferred to light; mean ± S.D., n = 3)
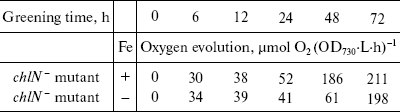
An interesting observation was the gradual blue shift of PS I peak upon greening under iron stress. This change in PS I fluorescence emission is even similar to that during the time course of wild-type transferred to iron-deficient conditions. Our results suggest that newly synthesized PS I under iron stress might have normal structure as that of iron-sufficient cells and hence alter substantially.
Under iron stress, the 684 nm peak increased sharply when the 695 nm peak appeared. This suggests that the biogenesis of IsiA and CP47 upon greening began at almost the same time but was subsequent to that of PS I. The greening time lasts for 72 h under iron deficiency, and lasts for about 48 h under iron sufficiency. As each IsiA subunit binds about 16-17 Chl molecules [21], abundance of IsiA may compete with PS II in binding newly synthesized Chl. Therefore, the delay in biogenesis of PS II under iron stress might be due to the presence of a large amount of IsiA.
In conclusion, the experimental system described here has opened up an opportunity to study the biogenesis of Chl-binding proteins in vivo under iron stress. The gradual blue shift of PS I peak upon greening under iron stress suggests the structural alteration of newly synthesized PS I. Furthermore, the delayed biogenesis of PS II might result from the large amount of IsiA under iron stress.
We thank Professor Jindong Zhao at College of Life Science, Peking University, for technical help on the low temperature fluorescence spectrometry.
This work was supported by the NSFC projects 40272054 and NSFC key project 40332022.
REFERENCES
1.Guikema, J. A., and Sherman, L. A. (1983) Plant
Physiol., 73, 250-256.
2.Sandmann, G., Peleato, M. L., Fillat, M. F.,
Lazaro, M. C., and Gomez-Moreno, C. (1990) Photosynth. Res.,
26, 119-125.
3.Sandstrom, S., Ivanov, A. G., Park, Y. I., Oquist,
G., and Gustafsson, P. (2002) Physiol. Plant., 116,
255-263.
4.Falk, S., Samson, G., Bruce, D., Huner, N. P. A.,
and Laudenbach, D. E. (1995) Photosynth. Res., 45,
51-60.
5.Burnap, R. L., Troyan, T., and Sherman, L. A.
(1993) Plant Physiol., 103, 893-902.
6.Bibby, T. S., Nield, J., and Barber, J. (2001)
Nature, 412, 743-745.
7.Boekema, E. J., Hifney, A., Yakushevska, A. E.,
Piotrowski, M., Keegstra, W., Berry, S., Michel, K. P., Pistorius, E.
K., and Kruip, J. (2001) Nature, 412, 745-748.
8.Park, Y. I., Sandstrom, S., Gustafsson, P., and
Oquist, G. (1999) Mol. Microbiol., 32, 123-129.
9.Sandstrom, S., Park, Y. I., Oquist, G., and
Gustafsson, P. (2001) Photochem. Photobiol., 74,
431-437.
10.Michel, K. P., and Pistorius, E. K. (2004)
Physiol. Plant., 120, 36-50.
11.Beale, S. I. (1994) in The Molecular Biology
of Cyanobacteria (Bryant, D. A., ed.) Academic Publishers,
Dordrecht, pp. 519-558.
12.Wu, Q. Y., and Vermaas, W. (1995) Plant Mol.
Biol., 29, 933-945.
13.Wu, Q. Y., Yu, J. J., Zhao, N. M., and Vermaas,
W. (1999) Tsinghua Sci. Technol., 4, 1544-1550.
14.Yu, J. J., Wu, Q. Y., Mao, H. B., Zhao, N. M.,
and Vermaas, W. (1999) IUBMB Life, 48, 625-630.
15.Rippka, R., Deruelles, J., Waterbury, J. B.,
Herdman, M., and Stanier, R. Y. (1979) J. Gen. Microbiol.,
111, 1-61.
16.MacKinney, G. (1941) J. Biol. Chem.,
40, 315-322.
17.Xu, H., Vavilin, D., Funk, C., and Vermaas, W.
(2002) J. Biol. Chem., 279, 27971-27979.
18.Ryberg, M., and Sundqvist, C. (1991) in
Chlorophylls (Scheer, H., ed.) CRC Press, Boca Raton, FL, pp.
587-612.
19.Sidler, W. A. (1994) in The Molecular Biology
of Cyanobacteria (Bryant, D. A., ed.) Kluwer Academic Publishers,
Dordrecht, pp. 139-216.
20.Van Dorssen, R. J., Breton, J., Plijter, J. J.,
Satoh, K., van Gorkom, H. J., and Amesz, J. (1987) Biochim. Biophys.
Acta, 893, 276-274.
21.Andrizhiyevskaya, E. G., Schwabe, T. M., Germano,
M., D'Haene, S., Kruip, J., van Grondelle, R., and Dekker, J. P. (2002)
Biochim. Biophys. Acta, 1556, 265-272.