Adhesion of Human Neutrophils to Fibronectin Is Inhibited by Comaruman, Pectin of Marsh Cinquefoil Comarum palustre L., and by Its Fragments
S. V. Popov, R. G. Ovodova, G. Yu. Popova, I. R. Nikitina, and Yu. S. Ovodov*
Institute of Physiology, Komi Science Center, The Urals Branch of the Russian Academy of Sciences, ul. Pervomaiskaya 50, 167982 Syktyvkar, Russia; fax: 7 (8212) 241-001; E-mail: ovoys@physiol.komisc.ru* To whom correspondence should be addressed.
Received July 22, 2004; Revision received August 30, 2004
Earlier, we detected antiinflammatory action of comaruman, pectin of the marsh cinquefoil Comarum palustre L. This effect can be explained by new data concerning inhibition of adhesion of human neutrophils to fibronectin by comaruman and its fragments. The galacturonan backbone fragment of molecular mass >10 kD appears to be the active region of the comaruman macromolecule. Comaruman CP (50-200 µg/ml) was found to decrease adhesion of neutrophils stimulated by phorbol 12-myristate 13-acetate (PMA, 1.625 µM) and by dithiothreitol (DTT, 0.5 mM). The fragments of comaruman CP-H (100 kD), CP-H1 (10-50 kD), and CP-H2 (100 kD) obtained by acidic hydrolysis and representing regions of linear polygalacturonan are shown to inhibit neutrophil adhesion more than the crude pectin. A fragment CP-E (<10 kD) obtained using pectinolysis and representing a branched region of the comaruman macromolecule failed to influence cell adhesion. The parent comaruman CP as well as fragments of its polygalacturonan backbone diminish PMA-initiated generation of oxygen radicals in neutrophils.
KEY WORDS: pectins, Comarum palustre, comaruman, linear polygalacturonan, neutrophil adhesion, fibronectin
Pectic polysaccharides have been shown to possess an immunomodulating and antiinflammatory effect without clear mechanism [1]. The marsh cinquefoil Comarum palustre L. (Rosaceae) has been widely used in ethnomedicine as a raw material for production of analgesic and antiinflammatory remedies [2]. Earlier, we isolated from the aerial part of C. palustre L. a pectin named comaruman and have detected its antiinflammatory action [3]. As known, neutrophil adhesion represents a key step of the inflammatory response [4]. In this connection, the antiinflammatory effect of comaruman was suggested to be connected with neutrophil interaction. Pectic polysaccharides have been established to have irregular block character of carbohydrate chain and to consist of macromolecular fragments of linear and branched areas. The linear part is represented by the segments of alpha-1,4-D-galacturonan, to make up the obligatory backbone of pectins. The ramified regions of pectins are composed by rhamnogalacturonan with the side chains formed by residues of neutral monosaccharides [1]. The segment of the pectic macromolecule that mediates the physiological activity has not yet been established.
The aim of the present investigation was the study of the effect of comaruman and its fragments on adhesion of human neutrophils to fibronectin.
MATERIALS AND METHODS
Reagents. Bovine serum albumin (BSA), phorbol 12-myristate 13-acetate (PMA), trypan blue, EDTA, fetal calf serum (FCS), and Hanks' solution were from ICN Biomedicals (USA); dextran T-500 and Ficoll-Paque from Amersham Pharmacia Biotech (Sweden); nitrotetrazolium blue (NBT), zymosan A, fucoidan from Fucus vesiculosis, and fibronectin of human blood plasma from Sigma (USA); dithiothreitol (DTT) from Loba Feinchemie AG (Austria).
Isolation of comaruman and preparation of its fragments. Comaruman CP was extracted from the fresh aerial part of C. palustre with aqueous ammonium oxalate as described earlier [5].
Acidic hydrolysis. Trifluoroacetic acid (TFA) (20 ml, 2 M) was poured into the polysaccharide fraction (100 mg). The resulting mixture was heated at 100°C for 5 h. The unreacted residue of galacturonan was separated by centrifugation, dissolved in water adjusting pH to 4-5 with aqueous ammonia under rigorous mixing, and the solution was dialyzed and lyophilized. Galacturonan (20 mg) (fraction CP-H) was obtained.
Enzymatic hydrolysis. The parent comaruman CP was preliminarily demethoxylated, using saponification with a solution of sodium methylate in absolute methanol (1.5 mg/ml, pH 10). Saponified polysaccharide (50 mg) was dissolved in water (5 ml) and 4 mg of pectinase (Fluka, Germany, activity 500 U/mg) was added, and the solution was digested at 37°C (pH 5). Enzymic hydrolysis was monitored by the increasing quantities of the reducing sugars in accord with the procedure of Nelson and Somogyi [6]. Pectinase was deactivated by boiling at 100°C, and the precipitate obtained was removed by a centrifugation. The solution was concentrated and saccharides were precipitated with 4 volumes of 96% ethanol. The precipitate was separated by centrifugation, dissolved in distilled water, and lyophilized to yield fraction CP-E (14 mg).
The CP-H fragment was separated using ultrafiltration membranes (Millipore, USA) to yield fractions CP-H1 and CP-H2 with molecular weight 10-50 kD and more than 100 kD, respectively.
General methods. Galacturonic acid was quantified with 3,5-dimethylphenol in the presence of concentrated H2SO4 [7] (the standard curve was constructed for D-galacturonic acid). Descending paper chromatography was carried out on Filtrak (Germany) FN-12 and FN-13 papers in 6 : 4 : 3 (v/v) n-butan-1-ol-pyridine-water system; spots of monosaccharides were detected by aniline hydrogen phthalate at 105°C. Neutral monosaccharides were qualitatively and quantitatively determined as the corresponding alditol acetates by GLC [8].
The absolute configurations of monosaccharides of comaruman were determined using GLC of acetylated (+)- and (-)-2-octyl glycosides as described earlier [9] in comparison with the corresponding glycosides of D-galactose, L-rhamnose, and L-arabinose as the authentic samples.
The general chemical characteristics of the parent comaruman CP and its fragments are given in Table 1.
Table 1. Yield and sugar composition of
comaruman and its fragments
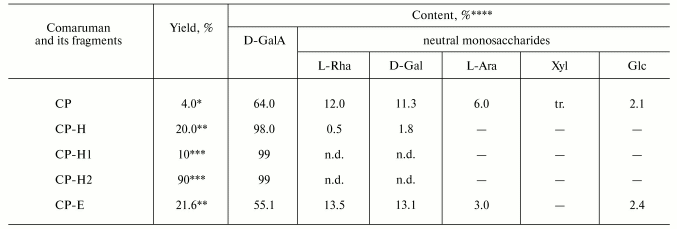
*Of air-dried raw material.
**Of the parent comaruman CP.
***Of fraction CP-H.
****Contents of D-galacturonic acid and neutral sugars are
determined in wt. %. Designations: tr., trace; n.d., not determined.
Neutrophil suspension. Neutrophils were isolated from peripheral blood of male volunteers and stabilized with 2.7% of EDTA. Erythrocytes were precipitated using 3% solution of dextran T-500. The leukocyte suspension was centrifuged at 400g in a density gradient of Ficoll-Paque (1.077-1.078 g/cm3). Neutrophils were collected from the deposit, washed thrice with saline, and resuspended in Hanks' solution supplemented with FCS (10%) to reach concentration of 3*106 cells/ml. The yield of neutrophils was 92 ± 5% [10].
Preparation of fibronectin-coated wells. Fibronectin was diluted in NaHCO3/Na2CO3 buffer (pH 9.6) to a concentration of 100 µg/ml and filled (100 µl) in the wells of 96-well medium protein binding Costar plates (Corning Inc., USA). Coating was allowed to proceed for 15 h at a room temperature. The sites of nonspecific binding were blocked with 1% solution of BSA (60 min, 37°C), and the wells were washed thrice with saline [11].
Measurement of adhesion. The polysaccharides samples (50 µl) were added to neutrophil suspension (100 µl) and were incubated in the fibronectin-coated plates at 37°C in the presence or absence of known cell stimulators--PMA (1.625 µM) and DTT (0.5 mM). Neutrophil adhesion of each donor was measured by scoring of number of non-adherent cells using light microscopy. The number of adherent neutrophils was determined by a colorimetric method using Giemsa dye. Optical density (A) of stained cell monolayer was measured after complete dissolution at 650 nm using a PowerWave 200 spectrophotometer (BioTek Instruments, USA) [10].
Estimation of respiratory burst. Generation by neutrophils of reactive oxygen species (ROS) was determined spectrophotometrically using the NBT-reducing test [12]. Neutrophil suspension (2*106 cells/ml) was incubated with polysaccharide solutions (100 µg/ml) in the presence or in the absence of stimulators, PMA (0.5 µg/ml) or zymosan (1 mg/ml), for 15 min at 37°C. A solution of 0.1% NBT (100 µl) was added into the wells and incubated at 37°C for 60 min. Optical density of solution was determined spectrophotometrically at 590 nm.
Cell viability was evaluated using 0.1% solution of trypan blue [13].
Statistics. The data were processed to obtain the arithmetic mean and the standard root-square-deviation. The reliability of differences was evaluated using Student's t-test.
RESULTS
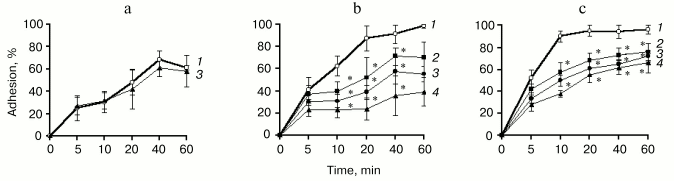
Sixty percent of neutrophils injected into the wells were found to attach spontaneously to plastics covered by fibronectin within 60 min (Fig. 1a). Cell adhesion was shown to increase up to 100% in the presence of PMA and DTT (Figs. 1b and 1c). Fucoidan (10 µg/ml) used as a known inhibitor of adhesion mediated by L-selectin failed to influence the number of adherent cells, indicating the “firm” adhesion of neutrophils.
Spontaneous adhesion of neutrophils to fibronectin was not changed in the presence of comaruman CP (Fig. 1a), whereas comaruman CP at concentrations more than 50 µg/ml was found to diminish neutrophil adhesion stimulated by PMA or DTT (Figs. 1b and 1c). The inhibitory effect of comaruman was displayed after 10 min of co-incubation with activated cells and was maintained during 60 min. Numbers of adherent neutrophils were found to decrease twofold in the presence of comaruman at the concentration of 100 µg/ml.Fig. 1. Inhibitory effect of comaruman CP on the adhesion of human neutrophil to fibronectin. The cells were co-incubated with comaruman (50 (2), 100 (3), 200 µg/ml (4)) in the absence (spontaneous adhesion) (a) or in the presence of activators--PMA, 0.5 µg/ml (b) and DTT, 0.5 mM (c). The number of adherent cells is expressed as a percentage of cells number injected into the well. The data represent arithmetic mean and standard deviation; n = 6; *, differences are significant versus control (1) at p < 0.05.
The fragment CP-H obtained by acidic hydrolysis of comaruman was established to inhibit cell adhesion in a greater extent than the parent pectin. Fragment CP-H at the concentration of 100 µg/ml was found to decrease fourfold the number of adherent cells stimulated with PMA (Table 2). The effect of the fragment CP-H on adhesion activated with DTT is comparable with that of the parent pectin. The galacturonan fragments CP-H1 and CP-H2 with molecular weights of 10-50 kD and more than 100 kD, respectively, were shown to reduce a number of adherent neutrophils. Both fragments are comprised in the composition of fragment CP-H. Fragment CP-E obtained by pectinase digestion of comaruman and possessing molecular weight less than 10 kD failed to influence neutrophil adhesion.
Table 2. Effect of fragments of comaruman on
adhesion of human neutrophils to fibronectin
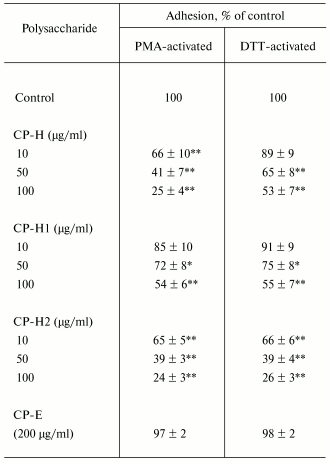
Note: Time of incubation is 20 min. The data represent the
arithmetic mean ± standard deviation; n = 6; * and
**, differences are significant versus control at p < 0.01
and p < 0.001, respectively.
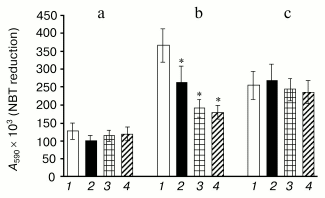
The parent comaruman CP as well as fragments (CP-H, CP-H1, and CP-H2) of its polygalacturonan generated by acidic hydrolysis were found to decrease the generation of oxygen radicals in neutrophils induced by PMA (Fig. 2). At the same time, the studied polysaccharides failed to activate self-maintained cells and influence the generation of ROS induced by zymosan.
The studied polysaccharides have no cytotoxic action. Cell viability was estimated to be equal to 92 ± 7 and 85 ± 7% in the control wells (60 min at 37°C) and in the presence of the polysaccharides (1000 µg/ml), respectively.Fig. 2. Effect of comaruman CP (2) and its fragments CP-H1 (3) and CP-H2 (4) on generation of reactive oxygen species by human neutrophils in the absence (a) or in the presence of PMA (0.5 µg/ml) (b) and zymosan (1 mg/ml) (c) (n = 6); *, differences are significant versus control (1) at p < 0.01.
DISCUSSION
Fibronectin is well known to be a protein of intercellular matrix that is extensively expressed during inflammation. In this connection, an influence of comaruman on adhesion of human neutrophils to fibronectin in vitro was studied to elucidate the mechanism of the antiinflammatory effect of this pectin. The firm adhesion of neutrophils to fibronectin has been earlier shown to be mediated by beta1- and beta2-integrins [11]. Comaruman CP was found to inhibit attachment to fibronectin of PMA- and DTT-stimulated neutrophils, not influencing non-activated cells. As known, increase in adhesion induced by PMA is caused by an augmentation of amounts of beta-integrins on the cell surface as a result of their mobilization from intercellular vesicles [14]. DTT does not change the number of expressed receptors but enhances its affinity to substrate [15]. Comaruman is suggested to bind with functionally active beta-integrins on the neutrophil surface and to prevent its interaction with fibronectin.
The parent comaruman CP was fragmented using acidic and enzymatic hydrolysis to elucidate what part of the pectic macromolecule was involved in the interaction with the cells. Polygalacturonan CP-H deprived of side carbohydrate chains as a result of acidic hydrolysis was established to decrease adhesion of neutrophils, whereas fragment CP-E obtained using digestion with pectinase failed to possess such capability. Therefore, comaruman inhibits neutrophil adhesion due to binding a fragment of the galacturonan backbone with the adhesion receptors. Fragment CP-H was shown to represent one-fifth of the whole pectic macromolecule (yield 20%, see Table 1). Therefore, more expressed inhibition effect of polygalacturonan CP-H in comparison with that of the parent pectin CP appeared to be elucidated by an increase in the concentration of the active segment of the molecule. The fragment of polygalacturonan with molecular weight of 10-50 kD appeared to be the minimum region of comaruman that possesses the inhibition action. Cleavage of comaruman into shorter fragments as during pectinase digestion was found to lead to a loss of inhibition capability.
Binding of beta-integrins with ligands is known to induce a functional activation of neutrophils followed by augmentation of cell response on subsequent stimulation [16]. Generation of ROS by neutrophils under the action of PMA is considerably reduced due to comaruman preventing the interaction of integrins with the adhesion substrate. The data suggest that the pectin binds and modifies surface receptors but fails to inhibit activation of the cell.
Integrin receptor of neutrophils is well known to consist of alpha and beta_polypeptide chains. Each chain can bind with polysaccharides. The lectin domain of the alpha-chain participates in recognition by leukocytes of endogenous glycoproteins, heparin, and microbial polysaccharides including zymosan [17]. The interaction of carbohydrate biopolymers with the lectin site is accompanied by an activation of the cell. Comaruman appeared not to bind with the sugar-recognizing domain of the alpha-chain of the integrin because comaruman failed to inhibit generation of ROS initiated by zymosan. The galacturonan fragment of comaruman is assumed to interact with beta-chain of integrins. Bacterial capsular polysaccharides containing glucuronic acid were earlier shown to be able to inhibit adhesion of neutrophils [18]. An inhibition effect of capsular polysaccharides at concentration of 100 µg/ml is caused by its interaction with beta-chain of integrins [19]. The ability of beta-integrins to interact with pectic polysaccharides has not been reported earlier.
Thus, an inhibitory effect of comaruman on adhesion of neutrophils to fibronectin was detected, explaining the antiinflammatory action of this pectin. A fragment of the galacturonan backbone with molecular weight more than 10 kD is assumed to be the active region of the comaruman macromolecule.
This work was supported by a grant of Leading Scientific Schools (No. SS-1260.2003.4), by the Russian Foundation for Basic Research (grant No. 03-04-48136), and by Programs of the Presidium of the Russian Academy of Sciences “Fundamental Sciences for Medicine” and “Molecular and Cellular Biology”.
REFERENCES
1.Ovodov, Yu. S. (1998) Russ. J. Bioorg.
Chem., 42, 483-501.
2.Kiosev, P. A. (2001) Comprehensive Catalogue of
Medicinal Plants [in Russian], EKSMO-Press, Moscow.
3.Ovodov, Yu. S., Ovodova, R. G., and Popov, S. V.
(2003) in Problems of Building of New Pharmaceuticals
(Abdrakhmanov, I. B., ed.) Gylem, Ufa, pp. 17-18.
4.Kuznetsov, V. F., and Chereshnev, V. A. (1998)
Pathophysiology of Neutrophil Dysfunctions [in Russian],
Kirov.
5.Ovodova, R. G., Vaskovsky, V. E., and Ovodov, Yu.
S. (1968) Carbohydr. Res., 6, 328-332.
6.Hodge, J. E., and Hofreiter, B. T. (1962) in
Methods in Carbohydrate Chemistry (Whistler, R. L., Wolfrom, M.
L., Bemiller, J. N., and Shafizadeh, F., eds.) Academic Press, New
York, pp. 380-394.
7.Usov, A. I., Bilan, M. I., and Klochkova, N. G.
(1995) Bot. Marina, 38, 43-51.
8.York, W. S., Darvill, A. G., McNeil, M., and
Stevenson, T. T. (1985) Meth. Enzymol., 118, 3-40.
9.Leontein, K., Lindberg, B., and Lönngren, J.
(1978) Carbohydr. Res., 62, 359-362.
10.Pinegin, B. V., Butakov, A. A., and Shchel'tsyna,
T. L. (1995) in Ecological Immunology (Khaitov, R. M., Pinegin,
B. V., and Istamov, H. I., eds.) [in Russian], VNIRO, Moscow, pp.
106-162.
11.Xu, X., and Hakansson, L. (1999) J. Immunol.
Meth., 226, 93-104.
12.Toni, S. D., Piva, E., Lapolla, A., Fontana, G.,
Fedele, D., and Plebani, M. (1997) Ann. N.-Y. Acad. Sci.,
832, 363-367.
13.Adams, R. (1983) The Methods of Cell Culture
for Biochemists [Russian translation], Mir, Moscow.
14.Sengelov, H., Kjeldsen, L., Kroeze, W., Berger,
M., and Borregaard, N. (1994) J. Immunol., 153,
804-810.
15.Yan, B., and Smith, J. W. (2001)
Biochemistry, 40, 8861-8867.
16.Schnitzler, N., Haase, G., Podbielski, A.,
Lutticken, R., and Shweizer, K. G. (1999) Nature Med., 5,
231-235.
17.Thornton, B. P., Vetvicka, V., and Pitman, M.
(1996) J. Immunol., 156, 1235-1246.
18.Dong, Z. M., and Murphy, J. W. (1997)
Infection Immunity, 65, 557-563.
19.Ellerbroek, P. M., Hoepelmann, A. M., Wolbers,
F., Zwaginga, J. J., and Coenjaerts, F. E. (2002) Infection
Immunity, 70, 4762-4771.