REVIEW: Actomyosin Systems of Biological Motility
In memory of my teacher Boris F. Poglazov
D. I. Levitsky1,2
1Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow 119992, Russia2Bach Institute of Biochemistry, Russian Academy of Sciences, Leninsky pr. 33, Moscow 119071, Russia; fax: (7-095) 954-2732; E-mail: levitsky@inbi.ras.ru
Received July 9, 2004
Evolution of notions on the molecular mechanism of muscle contraction and other events based on the actin-myosin interaction, from the middle of XX century to the present time, is briefly reviewed, including recent views on the functioning of the myosin head as a “molecular motor”. The results of structural and functional studies on the myosin head performed by the author and his colleagues using differential scanning calorimetry are also reviewed.
KEY WORDS: biological motility, myosin, actin, actomyosin, differential scanning calorimetry
Abbreviations: DSC) differential scanning calorimetry; S1) myosin subfragment 1; S2) myosin subfragment 2; HMM) heavy meromyosin; LMM) light meromyosin; Vi) orthovanadate anion; BeFx, AlF4-) beryllium fluoride and aluminum fluoride anions, respectively.
The cyclic interaction of myosin heads with actin filaments, which is
accompanied by ATP hydrolysis in the heads, is the basis of the
molecular mechanism of a number of events in biological motility, from
intracellular transport to muscle contraction.
One of the purposes of this review is to describe the evolution of notions on the molecular mechanism of motility realized by actomyosin systems. Undoubtedly, the founder of this field of science was V. A. Engelhardt, who discovered the ability of myosin, the main muscle protein, to hydrolyze ATP. This work published in Nature in 1939 [1] initiated a new scientific direction--mechanochemistry of muscle contraction, and predetermined the whole subsequent progress of muscle biochemistry.
Boris F. Poglazov played an important role in development of this field of science. Starting his scientific activities from studies on structure and properties of muscle myosin in the course of his diploma work and following preparation of the PhD thesis under the supervision of V. A. Engelhardt, later on he attempted, for the first time in the world, to find myosin in non-muscle cells and tissues. He was the first scientist who found myosin in non-muscle tissues of animals, as well as myosin-like proteins in higher plants and algae, and proposed from this finding that myosin is the main “motile” protein not only in muscles, but also in all eucaryotic cells. These innovative works of B. F. Poglazov were reflected in his monograph “Structure and Functions of Contractile Proteins” published in Russian by Nauka Publisher (Moscow) in 1965 [2] and in English by Academic Press (N.Y.) in 1966 [3]. This book for many years was a handbook for scientists working in this field. A hypothesis advanced many years ago by B. F. Poglazov, on the presence of myosin-like proteins in all eucaryotic cells, was very audacious and unusual for that time, but later on it was completely corroborated. At present, there is no doubt that just ATP-dependent interaction of myosin with actin is a universal molecular mechanism providing a number of various motility events in living cells.
I directly connect the beginning of my scientific activities with the name of my teacher Boris F. Poglazov. Under his supervision I performed my first studies on structure and properties of muscle myosin, and in 1982 we published together the monograph “Myosin and Biological Motility” [4].
In this review dedicated to my teacher Boris F. Poglazov, I will try to describe the evolution of notions on myosin as a “motor” protein, and to summarize the results of structural and functional studies of the myosin head obtained in our scientific team using differential scanning calorimetry.
EVOLUTION OF NOTIONS ON THE MOLECULAR MECHANISM OF MOTILITY IN
ACTOMYOSIN SYSTEMS
The term “myosin” originated as long ago as the XIX century (the term was introduced for the first time by W. Küne in 1864 and later on it was used by A. Ya. Danilewsky in 1881). The protein called myosin was extracted from muscle by high-ionic-strength solutions and precipitated upon decrease in ionic strength. In 1942-1943, F. B. Straub showed that myosin is a complex of two proteins; one of them was named actin, and the other retained the initial name--myosin. The actin-myosin complex was named actomyosin.
In 1939, V. A. Engelhardt and M. N. Ljubimova found that actomyosin (named myosin at that time) possesses ATPase activity [1]. Later on, they showed that addition of ATP to synthetic actomyosin filaments, obtained by blowing the actomyosin solution into water through a capillary, leads not only to ATP hydrolysis but also to a shortening of the filaments [5, 6]. Almost simultaneously, in 1942, A. G. Szent-Gyorgyi also observed the shortening of actomyosin filaments in ATP-containing solutions; later on, he also showed that glycerinated muscle fibers shorten upon ATP addition [7]. The main result of these works was the conclusion that muscle contraction is based on the interaction of actomyosin with ATP. These discoveries initiated studies on the molecular mechanism of muscle contraction.
In 1954, Andrew Huxley and Hugh Huxley formulated, independently from each other, the theory of muscle contraction [8, 9]. According to this theory, the contraction occurs due to mutual sliding of myosin and actin filaments, with the length of the filaments remaining unchanged. In ensuing years, intensive research was initiated on studies of structure and properties of the main muscle proteins--myosin and actin. Let us consider briefly what was known about the properties of these proteins and their interaction by the middle 1970s.
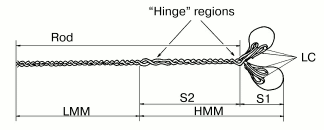
It was established by numerous studies that the molecule of muscle myosin is a hexamer comprising two heavy chains (molecular weight ~200 kD) and four light chains (molecular weight ~20 kD). The N-terminal parts of the heavy chains form two globular heads, with two light chains associated with each head. Each myosin head contains the ATPase site and the actin-binding sites. The C-terminal parts of the myosin heavy chains interact with each other and form a long and rigid rod part of the molecule (tail), which is a double alpha-helix. This double-coiled coil helix is formed due to periodic repeats of hydrophobic and charged residues. By limited proteolysis of the myosin molecule with trypsin, chymotrypsin, or papain, various fragments can be obtained in the isolated states: isolated myosin head or myosin subfragment 1 (S1); myosin rod; N- and C-terminal fragments of the myosin rod--subfragment 2 (S2) and light meromyosin (LMM), respectively; heavy meromyosin (HMM) consisting of two heads attached to S2 (Fig. 1) [4, 10]. These fragments, retaining the properties of some parts of the myosin molecule, are often used in experiments instead of intact myosin.
At that time, the mechanism of myosin Mg2+-ATPase reaction, including the changes not only of the nucleotide but also of the protein, was already investigated [11]:Fig. 1. Schematic representation of the molecule of muscle myosin (myosin II) and its fragmentation by proteolytic enzymes [4]: S1, myosin subfragment 1; S2, myosin subfragment 2; HMM, heavy meromyosin; LMM, light meromyosin; LC, light chains; Rod, rod part of the molecule. “Hinge” regions with low content of alpha-helix provide a high mobility of the myosin heads relative to the rod part and of the HMM region relative to the LMM region; these sites are the most sensitive to proteolysis, allowing the preparation of the isolated fragments of the myosin molecule.
M + ATP <-> M-ATP <-> M*-ATP <-> M**-ADP-Pi <-> M*-ADP-Pi <-> M*-ADP + Pi <-> M-ADP <-> M + ADP,
where M is the active site of myosin, HMM, or S1; M* and M** denote isomeric forms of the protein with increased tryptophan fluorescence (each asterisk reflects the increase in the intrinsic fluorescence).
As to actin, it was established that in muscle it functions in the form of filamentous actin (F-actin), which is a double-stranded spiral polymer of actin monomers. Monomeric actin (G-actin) is a globular protein with molecular weight of 42 kD consisting of a single polypeptide chain. Each G-actin molecule contains bound ATP and a divalent cation. An important feature of actin is its ability to polymerization upon addition of neutral salts with formation of long polar filaments of F-actin.
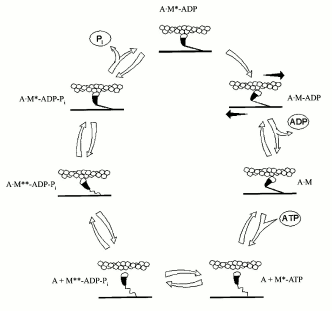
By that time, it was already clear that the basis of muscle contraction is a longitudinal movement (“sliding”) of myosin and actin filaments relative to each other, with no changes in the length of the filaments. The filaments are connected by “cross-bridges”--myosin heads, which protrude, together with S2 region, from the surface of myosin filament and can interact with actin. This interaction is coupled with ATP hydrolysis in the myosin heads. In the absence of ATP, the cross-bridges are strongly bound to actin filaments. However, when myosin heads bind ATP, their affinity to actin strongly decreases with the result that the cross-bridges detach from the actin filaments (Fig. 2). Then the ATP hydrolysis occurs in the active sites of myosin ATPase, and the M**-ADP-Pi intermediate is formed (see the scheme for myosin Mg2+-ATPase reaction shown above). Formation of this intermediate complex is accompanied by conformational changes in the myosin molecule (M**). In this state, the cross-bridges again bind to actin filaments, but very weakly and at another angle in comparison with the binding in the absence of ATP (Fig. 2). This binding strongly accelerates the process of isomerization of the M**-ADP-Pi intermediate and, correspondingly, the following release of the products of the ATPase reaction from the active site of myosin ATPase; in vitro this is expressed in a significant activation of the myosin Mg2+-ATPase by actin. Upon this isomerization, the orientation of the myosin head relative to actin is significantly altered, leading to movement of the actin filament along the myosin filament. Then myosin heads again bind ATP, dissociate from actin, and the cycle repeats again and again. As a result, a mutual “sliding” of myosin and actin filaments occurs relative to one another, which is the basis for muscle contraction.
By mid 1970s, it became clear that the molecular mechanism of muscle contraction is based on significant conformational changes occurring in the myosin molecule during ATPase reaction upon formation of the M*-ATP and M**-ADP-Pi intermediates. At that time, the main problem was to elucidate in which part of the myosin molecule these conformational changes occur, which lead to the movement of actin filaments along myosin filaments. Until the late 1980s, the hypothesis proposed by W. F Harrington [12] was very popular; according to this hypothesis, a rapid helix-to-coil transition of the “hinge” region between S2 and LMM in the myosin rod (Fig. 1) plays a key role in the force generation during muscle contraction. Therefore, at that time many investigators studied the myosin rod by many various methods. In particular, B. F. Poglazov with coauthors (I was among them) found that mechanical deformation (stretching) of the myosin rod part has an influence on the ATPase activity of myosin [4, 13]. Many other authors supposed that the site responsible for the force generation during muscle contraction is located in the other “hinge” region, between the heads and the rod part of myosin (Fig. 1), i.e., in the region providing a high mobility of the heads. Thus, at that time the site responsible for “motor” functions of myosin was believed to be located in any parts of the myosin molecule except for the head itself. This was mainly due to the fact that the only myosin known at that time was muscle myosin (or similar myosins) possessing complicated structure (Fig. 1) and functioning only in the form of well-ordered filaments. The complex structure of the rod part of muscle myosin caused many investigators to search for the site responsible for motor functions of myosin just in this part of the myosin molecule.Fig. 2. A scheme showing the working cycle of the myosin head on its interaction with actin filaments during muscle contraction. In all the states, the actin filament is represented above the myosin filament (A, actin; M, myosin head).
The situation changed by the late 1980s and the early 1990s. This was due to new data on the structure and properties of myosin. First, it became clear that myosins are highly diverse, and they are present not only in muscles but also in non-muscle cells. Second, it was established that just the myosin head is the universal “molecular motor”. Let us consider in more detail those achievements that led to the recent views on the molecular mechanism of motility in actomyosin systems.
Diversity of Myosins. “Unconventional” Myosins
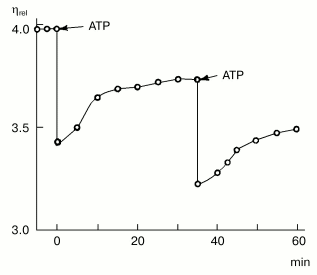
Myosin was found in non-muscle cells and tissues for the first time by B. F. Poglazov more than 40 years ago, in the early 1960s. In 1961, he prepared a myosin-like protein from bovine brain [14]. The protein possessed ATPase activity, and its viscosity increased upon addition of muscle F-actin. The most important evidence that the protein obtained from brain is indeed myosin was that its complex with F-actin reversibly dissociated upon ATP addition (this was one of the most characteristic features of muscle myosin). Addition of ATP led to a sharp decrease in the viscosity of the complex, with following recovery of viscosity in the course of ATP hydrolysis [14] (Fig. 3). Then B. F. Poglazov used this successful approach for identification of myosin-like proteins prepared from pancreas, thyroid, and liver [15], as well as from the alga Nitella flexilis [16]. He suggested from these data that myosin is present in all living cells [2, 3]. This suggestion, which was rather audacious for that time, was too unusual to be properly perceived by the scientific community, but later on it was completely corroborated.
By the end of the 1960s and in the early 1970s, purification of myosin from many different non-muscle cells was reported by many authors. All these proteins were similar to the muscle myosin in their structure and properties, i.e., they had two heads and the rod part responsible for formation of myosin filaments. However, in 1973, a myosin-like protein was purified by T. D. Pollard and E. D. Korn from Acanthamoeba castellanii, whose molecule consisted of only one heavy chain with molecular weight of 125-130 kD and one or two light chains [17]. This “one-headed” myosin was named myosin I, in distinction from usual two-headed myosin which was named myosin II. Myosin I is a globular protein devoid of the rod part. It is unable to form ordered filaments, but it shows all other properties characteristic for myosins--specific ATPase activity and ability to interact with actin, with activation of myosin ATPase by actin. It was shown that myosin I is not the product of proteolytic cleavage of myosin II, which was also purified from A. castellanii, and these two proteins are expressed by different genes [18]. Later on, a wide distribution of myosins I was demonstrated: it is now clear that these proteins are expressed in most if not all vertebrate tissues, and they play an important role in cell motility [19].Fig. 3. Effect of ATP (2.4 mM) on the viscosity of a mixture of brain myosin with muscle F-actin [14]. A decrease in viscosity (eta, relative units) of the actin-myosin complex with its following recovery in the course of ATP hydrolysis, which was a characteristic feature for muscle myosin, was the best evidence for that time (1961) that the protein prepared from bovine brain was indeed myosin. (The figure is taken from the monograph “Structure and Functions of Contractile Proteins” by B. F. Poglazov [3].)
In the 1990s, it became clear that myosin I is not the only “unconventional” myosin different from the “conventional” myosin II. Almost every year (or even a few times a year) new types of unconventional myosin have been found [20]. It is now clear that myosins represent a superfamily of 139 proteins, which are very diverse both in their structure and in their functions in cells. On the basis of phylogenetic analysis, all myosins are subdivided into 17 classes [21]. Myosins have one head (myosins I, III, IV, IX, and XIII) or two heads (myosins II, V, VI, VII, X, and XI) containing in the “neck” region from one to six binding sites for light chains or calmodulin. The C-terminal regions (tails) are very diverse both in size and sequence among different myosins [20, 22]. The specificity of individual myosin function in the cell is dictated by this variable part of the molecule. For example, the C-terminal part of myosin can contain membrane-binding sites or sites for ATP-independent binding to actin. Only myosins II, including all muscle myosins, have a long rod part providing formation of myosin filaments.
Unlike the C-terminal parts (tails), the globular N-terminal parts (heads) are highly conserved for all myosins. This means that just the myosin head, possessing the ATPase and the actin-binding sites, is the genuine “molecular motor”, i.e., the force generation process during actin-myosin interaction occurs within the head, not in some other parts of the myosin molecule.
Myosin Head as a Molecular Motor
In 1987, it was shown that isolated myosin heads (myosin subfragment 1, S1) are capable of moving actin filaments in an in vitro motility assay [23]. It became clear that just the myosin head is the molecular motor capable by itself of the motile functions. Therefore, the interest of most investigators studying myosin structure and functions switched to studies on the myosin head.
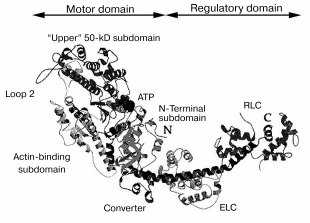
In 1993, the three-dimensional structure of the myosin head was determined by X-ray analysis of S1 crystals [24]. An important feature of this structure is the existence of clearly pronounced morphological domains in the myosin head, i.e., the motor domain and the regulatory domain. The motor domain represents the globular part of the head; it contains the ATPase site and the actin-binding sites. Approximately after the 780th residue, the motor domain turns into the regulatory domain, which is a long rigid alpha-helix stabilized by two noncovalently associated light chains--“essential” light chain and regulatory light chain (Fig. 4).
According to recent views, the functioning of the myosin head as a molecular motor is provided by a ~60° turning of the regulatory domain (also called “lever arm”) relative to the motor domain [25]. The regulatory domain acts as a lever whose length determines the size of displacement of the myosin head along the actin filament. Lengthening or shortening of the regulatory domain by inserting or deleting light-chain-binding-sites using mutagenesis led to an increase or decrease, respectively, in the speed of transport of actin filaments by immobilized S1 molecules in the in vitro motility assay [26]. It was found in similar experiments, when the regulatory domain was replaced by an artificial lever arm fabricated from alpha-actinin repeating units (rigid triple alpha-helix), that the speed of actin transport was proportional to the length of the lever arm [27]. It was shown that modifications of the regulatory domain, as well as its removal or replacement by alpha-actinin repeats, had no influence on the actin-activated ATPase of S1, i.e., these alterations to the lever arm length did not affect the rate of S1 cyclic work, but they did affect the size of displacement of actin filaments on hydrolysis of each ATP molecule.Fig. 4. A ribbon representation of the three-dimensional structure of the myosin head (S1) [24, 41]. Four subdomains of the motor domain of the head are indicated. “Loop 2” designates the location of a flexible loop between the “upper” and “lower” (actin-binding) 50-kD subdomains, which is not seen in the S1 crystals. ATP, nucleotide-binding site; ELC, “essential” light chain; RLC, regulatory light chain.
Thus, the efficiency of movement of the myosin head along the actin filament during one working cycle upon hydrolysis of one ATP molecule depends on the length of the lever arm--the regulatory domain of the head [25]. The size of this movement (“step size”) is 36 nm for myosin V [28], whose regulatory domain is three times longer than for conventional myosin II, i.e., it is bigger by 3-4 times than the step size for myosin II.
It is now clear that the main events leading to rotation of the regulatory domain relative to the motor domain occur mainly in the motor domain of the myosin head during ATP binding and hydrolysis and due to interaction with actin. The greatest conformational changes occur on formation of the intermediates M*-ATP and M**-ADP-Pi during the ATPase reaction. However, these intermediates exist during the ATPase reaction for a very short time, which is not enough for detailed structural studies. For this purpose stable analogs of these intermediates are successfully used, i.e., ternary complexes of the myosin head (S1) or its isolated motor domain with ADP and Pi analogs such as orthovanadate (Vi), beryllium fluoride (BeFx), or aluminum fluoride (AlF4-) anions [29-31]. It has been shown that the S1-ADP-BeFx complex is different from all other ternary complexes of S1 with ADP and Pi analogs: it resembles the S1*-ATP intermediate state, whereas the complexes S1-ADP-Vi and S1-ADP-AlF4- resemble the S1**-ADP-Pi intermediate state [32, 33].
These stable analogs of the myosin ATPase intermediates were successfully used for crystallographic studies of the myosin head. In 1995-1996, the atomic structures of the isolated motor domain of the head of Dictyostelium discoideum myosin II in the complexes with ADP-BeFx, ADP-AlF4- [34], and ADP-Vi [35] were solved. An intriguing result of these studies was that the structure of the motor domain of Dictyostelium myosin head with ADP-BeFx bound was remarkably similar to the corresponding part of the earlier solved structure of skeletal chicken S1 without nucleotide [24], whereas the complexes with ADP-AlF4- and ADP-Vi significantly differed in their structure from nucleotide-free S1 [34, 35]. This was in contradiction with numerous literature data showing that formation of the S1-ADP-BeFx complex induces significant conformational changes in the S1 molecule, similar to those caused by formation of the complexes S1-ADP-Vi and S1-ADP-AlF4- [30, 31, 36-38]. However, contrary results were obtained in 1998, when the atomic structures of smooth muscle myosin head in the complexes with ADP-BeFx and ADP-AlF4- were solved [39]. In both these complexes, the structure of S1 was almost identical, but it was quite different from the structure of nucleotide-free skeletal S1. The main difference was that the C-terminal region of the motor domain (called the “converter”, Fig. 4), connecting the motor domain with the regulatory domain, has been rotated by about 70o from its position seen in the nucleotide-free S1 [39]. This rotation of the converter led to a movement of the lever arm. Thus, structural evidence has been obtained for the “lever-arm model” explaining the molecular mechanism of functioning of the myosin “motor”.
One year later, in 1999, studies of the atomic structure of scallop S1 revealed another conformational state of S1, i.e., S1 in the complex with ADP [40]. In this state the converter (and, correspondingly, the lever arm) has been rotated by about 30° from the position seen in the nucleotide-free S1, in the opposite direction in comparison with the complexes S1-ADP-Vi and S1-ADP-AlF4-. Thus, three main conformational states of the myosin head (S1) differing from each other in the position of the converter in the motor domain of the head and, correspondingly, in the position of the regulatory domain (lever arm) relative to the motor domain, are now considered: S1 without nucleotide, S1-ADP, and S1 in stable ternary complexes with ADP and Pi analogs (Vi, AlF4-, and BeFx) which mimic the S1 ATPase intermediates S1*-ATP and S1**-ADP-Pi [41]. Just the transitions of the myosin head from one conformational state to the other state during ATPase cycle, which are accompanied by the rotation of the lever arm, determine the work of the myosin head as the molecular motor.
The main goal of recent studies has been to understand how local conformational changes in the myosin ATPase site spread to the entire motor domain of the head, resulting in the global structural changes in the motor domain and the rotation of the lever arm. According to recent views, this process involves displacement or rotation of four subdomains in the motor domain of the head (the so-called “upper” 50-kD subdomain, “lower” 50-kD subdomain in recent time also called “actin-binding” subdomain, N-terminal subdomain, and converter) (Fig. 4). A few joints connecting the subdomains play a key role in their movement. These are the so-called “switch II” connecting the upper and the lower 50-kD subdomains at the bottom of the actin-binding cleft separating these subdomains, the “relay” connecting the lower (actin-binding) 50-kD subdomain to the converter, and two short alpha-helices with so-called “essential” SH-groups at their ends, SH1 and SH2 (residues Cys707 and Cys697, respectively, in skeletal S1); one of these helices (the so-called “SH1 helix”) connects the N-terminal subdomain to converter, and the other (“SH2 helix”) is located in the N-terminal subdomain close to the ATPase site, which is between the N-terminal subdomain and the upper 50-kD subdomain (Fig. 4) [40, 41].
However, it should be noted that analysis of the crystal structures of the myosin head in its different states could not answer a number of questions. For example, on this analysis the complex of the myosin head with ADP and BeFx (stable analog of the M**-ATP intermediate of myosin ATPase reaction) either is similar in structure to the nucleotide-free S1 [34], or it is identical with the S1-ADP-AlF4- complex (analog of the M**-ADP-Pi intermediate) [39]. This is in contradiction with numerous literature data obtained by other methods, which demonstrate that the complex S1-ADP-BeFx significantly differs both from the S1-ADP-Vi and S1-ADP-AlF4- complexes, and from S1 without nucleotides or in the presence of ADP [30-33, 36-38]. However, it is important to note that in crystallographic studies the data obtained from different myosin isoforms or truncated constructs were often compared. For example, the crystal structures of the myosin head from Dictyostelium and from smooth muscles in the complexes with ADP-BeFx [34, 39] were compared with the structure of nucleotide-free skeletal S1 [24]. Moreover, crystallographic studies do not yet allow revealing and investigating the structural changes occurring in the myosin head due to interaction with actin. Such analysis has been carried out so far only by fitting the atomic structures of S1 and actin into three-dimensional cryoelectron microscope reconstructions of actin filaments decorated with S1 [42], i.e., it leaves out of account the actin-induced structural changes in the myosin head.
Studies of the myosin head as the molecular motor required to create a universal approach for probing (and then investigating in detail) the global structural changes that occur in the myosin head during ATPase reaction and due to interaction with actin. More than ten years ago, we offered such an approach based on an analysis of the thermal unfolding of the myosin head, as measured by differential scanning calorimetry (DSC).
STRUCTURAL AND FUNCTIONAL STUDIES OF THE MYOSIN HEAD USING
DIFFERENTIAL SCANNING CALORIMETRY
Differential scanning calorimetry (DSC) is the most effective and commonly employed method to study the thermal unfolding of proteins [43, 44]. Structural changes occurring in proteins upon modeling of processes of their functioning significantly affect the thermal unfolding of proteins. Therefore, the use of the DSC allows in some cases to obtain valuable (and sometimes even unique) information on conformational changes that occur in the protein molecule during its functioning.
Structural Changes Occurring in the Myosin Head during the ATPase Reaction
Since 1990 [45], we have successfully used DSC for studying structural changes occurring in the myosin head due to formation of stable ternary complexes with ADP and Pi analogs.
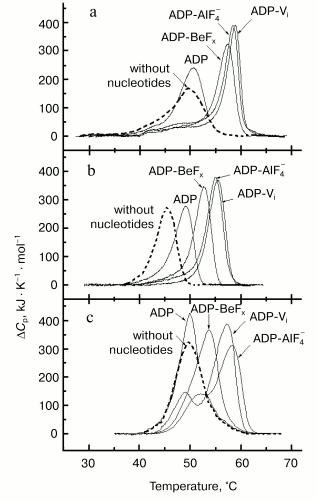
Figure 5a presents the heat sorption curves of skeletal S1 in the absence of nucleotides, in the presence of ADP, and in the ternary complexes S1-ADP-Vi, S1-ADP-AlF4-, and S1-ADP-BeFx [37, 46-49]. The binding of ADP to S1 does not significantly affect the temperature of the S1 thermal transition, but it increases the cooperativity of the transition (the peak becomes much narrower). On the other hand, the formation of the S1-ADP-Vi complex leads to considerable structural changes in the entire S1 molecule [46]: the maximum of the thermal transition shifts by almost 10°C to higher temperature, and the peak becomes very narrow, indicating a considerable increase in the cooperativity of the thermal transition. The calorimetric enthalpy of the transition also increases. The same effects were observed on formation of the S1-ADP-AlF4- complex. A similar though somewhat less pronounced effect was also observed on formation of the S1-ADP-BeFx complex (Fig. 5a).
Thus, DSC is very useful for probing the structural changes that occur in the myosin head due to formation of stable ternary complexes with ADP and Pi analogs. The use of various naturally occurring nucleoside diphosphates [38] and their synthetic non-nucleoside analogs [50, 51] showed that these changes revealed by DSC adequately reflect those changes which occur in the S1 molecule in the course of the ATPase reaction. It was also concluded from DSC experiments on recombinant fragments of Dictyostelium discoideum myosin II that the changes in the thermal unfolding, which are due to formation of stable ternary complexes with ADP and Pi analogs, occur mainly in the globular motor portion of the head [52]. Similar changes were also observed with HMM from smooth muscles of turkey gizzard containing dephosphorylated or fully phosphorylated regulatory light chains [53]. Thus, the presence of two heads in the HMM molecule and the extent of phosphorylation of the regulatory light chains have no significant influence on the structural changes induced in the globular motor portion of the head by the formation of stable ternary complexes with ADP and Pi analogs.Fig. 5. Temperature dependencies of excess heat capacity (DeltaCp) of rabbit skeletal S1 (a), M765 (the isolated motor part of the head of Dictyostelium myosin II) (b), and turkey gizzard smooth muscle HMM with dephosphorylated regulatory light chains (c) in the absence of nucleotides (dotted line), in the presence of ADP, and in the ternary complexes with ADP and Vi, AlF4-, or BeFx. Conditions: 30 mM Hepes, pH 7.3, 1 mM MgCl2. Heating rate 1 K/min.
In the case of skeletal S1 the changes in the thermal unfolding induced by formation of the S1-ADP-BeFx complex were similar to those observed for the complexes S1-ADP-Vi and S1-ADP-AlF4- although slightly less pronounced (Fig. 5a). However, a more pronounced difference between the complexes was observed by DSC studies with smooth muscle S1 and HMM [53], as well as with recombinant fragments of D. discoideum myosin II corresponding to the motor domain of the myosin head [52]. In these cases the effects of Vi and AlF4- were almost the same, whereas the effect of BeFx was much less pronounced: the shift in the protein thermal transition to higher temperature induced by formation of the complex with ADP-BeFx was much less, by 2.5-4°C, than that observed for the ternary complexes with ADP-Vi or ADP-AlF4- (Fig. 5, b and c). The most remarkable difference between the complexes was revealed by DSC studies when skeletal S1 was specifically modified at residues Lys83, Cys707, or Cys697 [48, 54, 55]. It was shown that these modifications prevent to a great extent the conformational changes of the S1 molecule resulting from the formation of the ternary complexes S1-ADP-Vi and S1-ADP-AlF4- (i.e., they prevent the shift of the S1 thermal transition to higher temperature), but they have almost no influence on the changes induced by the formation of the S1-ADP-BeFx complex. These results suggest that during formation of the S1-ADP-Vi and S1-ADP-AlF4- complexes the region containing Cys707, Cys697, and Lys83 (these residues are spatially located rather close to each other in the S1 molecule) plays an important role in transmission of structural changes from the active site of S1 ATPase to the entire motor portion of the myosin head, but this region does not take part in the transmission process on formation of the S1-ADP-BeFx complex.
Thus, the use of DSC (in combination with EPR [32]) revealed what crystallographic studies failed to do [34, 35, 39-41]: we have shown that the structure of the myosin head in the complex with ADP-BeFx significantly differs both from the structure in the absence of nucleotide or in the presence of ADP, and from that in the complexes with ADP-Vi or ADP-AlF4-. These data show that the complex of the myosin head with ADP-BeFx is a separate structural state of the head, which is different from the other states. This means that the myosin head can exist not only in three conformational states, as postulated from the data of crystallographic studies [41], but in four states: in the absence of bound nucleotide, in the complex with ADP, in the ternary complexes with ADP-Vi or with ADP-AlF4- (stable analogs of the M**-ADP-Pi intermediate of the myosin ATPase cycle), and in the complex with ADP-BeFx (stable analog of the M*-ATP intermediate).
Domain Structure of the Myosin Head and Its Changes during the ATPase Cycle
In the light of recent data on the atomic structure of the myosin head [39-41], analysis of domain structure of the head is of great interest because interdomain interactions might induce the internal motions in the head and play a key role in the transduction of energy of ATP hydrolysis into mechanical work.
The DSC method is one of the best approaches for revealing structural domains in multidomain proteins as distinct thermal transitions on the heat sorption curve. The most distinct and general feature of a domain in a globular protein is that its structure folds and unfolds cooperatively in an “all-or-none” way with significant changes in enthalpy and entropy [43]. In accordance with this definition, domains can be detected in DSC studies as the regions in the protein molecule that unfold cooperatively and independently from each other. To reveal such domains in the myosin head, in early works we applied the “successive annealing” method for DSC studies on the thermal unfolding of skeletal S1 [37, 46, 56-58]. This method based on the repetition of the cycle of “heating-cooling-heating to a higher temperature” is applied to fully or partially irreversible thermal transitions. The method permits experimental decomposition of the total heat sorption curve of a protein into elementary thermal transitions corresponding to the melting of separate structural domains in the protein molecule. By means of this approach, three such transitions (calorimetric domains) were revealed in the S1 molecule [46, 57, 58]. For identification of these domains (i.e., for revealing their correspondence to certain parts of the primary structure), many special approaches were applied. On the basis of results obtained and of their comparison with literature data, I proposed a domain model of the myosin head [59]. According to this model proposed for the first time in 1991 [60], the least thermostable and the most thermostable calorimetric domains reflect the melting of the “neck” region with associated light chains (later on named regulatory domain of the head), whereas the middle domain, comprising ~50% of the S1 enthalpy, corresponds to the globular (motor) portion of the head [48, 59]. Thus, the existence of domain structure in the myosin head was predicted from the DSC data two years earlier than the first data on the S1 atomic structure were published [24], which confirmed the existence of two clearly pronounced separate morphological domains in the myosin head, i.e., the motor domain and the regulatory domain (Fig. 4).
Recently we have proposed a new approach for analyzing the domain structure of irreversibly denaturing proteins from their DSC curves, which is suitable even in the case of overlapping peaks of separate calorimetric domains [61]. The DSC experiments are preceded by preliminary incubation of a protein at definite temperature for a definite time, the temperature for this incubation and its duration being determined from analysis of the DSC curves of a protein measured at different scanning rates. The parameters of thermal denaturation of separate calorimetric domains are verified by DSC experiments with such type treated protein at different scanning rates. The limited space of this review does not permit more detailed description of this rather complicated approach, and therefore I present here only the main results obtained from studies on the domain structure of the myosin head using this approach. Four separate calorimetric domains were revealed in rabbit skeletal S1, in good agreement with earlier DSC results obtained by the “successive annealing” method [46, 56-59]. However, only one calorimetric domain was revealed in the recombinant fragment M765 of the head of D. discoideum myosin II corresponding to the globular motor portion of the head that lacks the “neck” region and the light chains. By comparing these results we conclude that the whole motor domain of the myosin head unfolds as a single calorimetric domain (i.e., the DSC method does not permit dividing the motor domain into separate subdomains), while the other calorimetric domains comprising all together about half of the total enthalpy of the thermal unfolding of S1, can be assigned to thermal denaturation of the regulatory domain of the myosin head.
Surprisingly, only one calorimetric domain was revealed in the S1 molecule in the ternary complexes S1-ADP-Vi and S1-ADP-BeFx [61]. The results suggest that in these complexes, a tight coupling occurs between the motor and regulatory domains of the myosin head, and due to this interaction, both these parts of the head denature together as a single calorimetric domain. According to crystallographic data, formation of the S1-ADP-Vi complex leads to significant movement of the regulatory domain (lever arm) relative to the motor domain, and as a result the lever arm is located rather close to the motor domain surface in this complex [41]. The DSC results [61] indicate that in the complexes S1-ADP-Vi and S1-ADP-BeFx the regulatory domain and the motor domain not only locate close to each other due to rotation of the regulatory domain, but tight interaction occurs between both these domains of the myosin head. These results suggest that during the ATPase reaction the myosin head undergoes global changes in its domain structure, which are expressed in the tight coupling between the two main parts of the head, the motor domain and the regulatory domain.
Actin-Induced Structural Changes in the Myosin Head
As noted above, crystallographic studies do not yet allow revealing and investigating the structural changes that occur in the myosin head due to interaction with actin. For this purpose, we successfully use DSC.
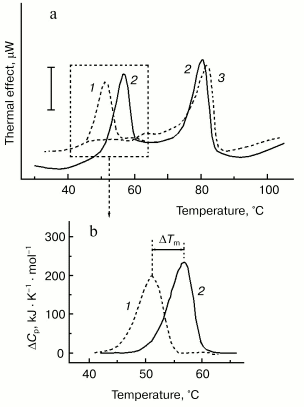
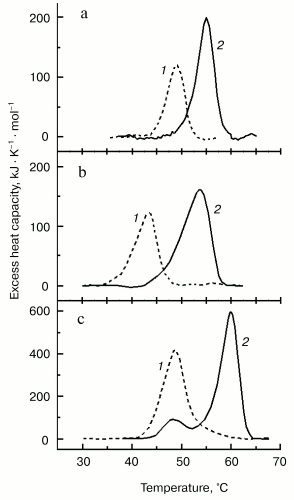
For better separation of thermal transitions of F-actin and actin-bound myosin head on the thermogram, we used in recent DSC experiments F-actin stabilized by phalloidin. In this case, F-actin melts at a very high temperature (of about 80°C), which allows more detailed study of the parameters of the thermal unfolding of the myosin head bound to F-actin (Fig. 6). The thermal unfolding of phalloidin-stabilized F-actin is not significantly affected by the interaction with S1 (Fig. 6a). In contrast, this interaction increases the thermal stability of S1. This actin-induced stabilization of the myosin head is expressed in a pronounced shift of its thermal transition to higher temperature (Fig. 6b). We use this shift (DeltaTm) as a relative measure for actin-induced structural changes in the myosin head. The size of DeltaTm depends on the source of myosin: it varies from 5-6°C for rabbit skeletal S1 (Fig. 6b) and D. discoideum myosin II head fragment M765 [62] (Fig. 7a) to 10-11°C for myosin I (Fig. 7b) and smooth muscle HMM (Fig. 7c). It has been concluded from similarity of the DeltaTm values for S1 and M765 that actin-induced changes in the thermal unfolding of the myosin head occur mainly in the globular motor portion of the head [62].
Fig. 6. Use of DSC for probing the actin-induced conformational changes in the myosin head [48, 62, 64, 69]. a) Experimental DSC profiles of rabbit skeletal S1 (1), F-actin stabilized by phalloidin (3), and their complex obtained in the presence of ADP (2). Conditions: 13 µM S1, 24 µM F-actin, 40 µM phalloidin, 20 mM Hepes, pH 7.3, 2 mM MgCl2, 1 mM ADP. The vertical bar corresponds to 15 µW. Heating rate 1 K/min. b) Excess heat capacity functions of S1 in the absence (1) and in the presence (2) of F-actin. The parameter DeltaTm (5.7°C in this case) is defined by the difference between denaturation temperatures (Tm) of the actin-free and actin-bound S1.
It has been shown that charge changes in the actin-binding surface loop (so-called “loop 2”, see Fig. 4) of the myosin head strongly affect the thermal unfolding of the myosin motor domain bound to F-actin [62]. Introduction of many additional negative changes into loop 2 of the isolated motor domain of the head of D. discoideum myosin II (M765) strongly decreased (from 6 to 1.2°C) the parameter DeltaTm, whereas addition of positively charged residues to the loop 2 produced a drastic increase, up to 9.1°C, of this parameter. All these mutant constructs did not significantly differ from each other in their ability to undergo global structural changes due to the formation of stable ternary complexes with ADP and Pi analogs [62]. Thus, the alterations in actin-binding loop 2 do not affect the nucleotide-induced structural changes in the myosin head, but they do affect the changes that occur in the motor domain of the head due to its strong binding to F-actin in the presence of ADP.Fig. 7. Temperature dependencies of excess heat capacity for M765 (the isolated motor part of the head of D. discoideum myosin II) (a), MyoIE700 (the isolated motor part of the head of D. discoideum myosin I) (b), and turkey gizzard smooth muscle HMM (c) in the absence (1) and in the presence (2) of a twofold molar excess of F-actin stabilized by phalloidin. In this figure a temperature region above 70°C, corresponding to the thermal denaturation of phalloidin-stabilized F-actin (Fig. 6a), is not shown. Heating rate 1 K/min. DeltaTm = 6°C (a), 10.7°C (b), and 11.2°C (c).
Direct electrostatic interaction between loop 2, a lysine-rich surface segment of the myosin head, and the negatively charged N-terminal part of actin is believed to be mainly responsible for the “weak” binding to F-actin of the myosin head complexed with ATP or ADP-Pi. The following transition to the strongly bound state (when myosin head loses Pi and contains only ADP) is accompanied by formation of many additional contacts between actin and myosin. This transition from the weakly bound state to the strongly bound state plays a crucial role in force generation during muscle contraction as it produces a movement of actin filaments along myosin filaments.
After detailed study of the thermal unfolding of myosin head strongly bound to F-actin, we applied the DSC approach described above to study the weak binding of S1 to F-actin.
“Weak” binding of pPDM-S1 to F-actin. The weakly bound states (binding to F-actin of the myosin heads in the complexes M*-ATP and M**-ADP-Pi) are short-lived intermediates of the actomyosin ATPase cycle; therefore, stable analogs of these states are required for structural studies. One of these analogs is rabbit skeletal S1 with SH-groups of Cys707 and Cys697 cross-linked by the bifunctional thiol reagent, N,N´-p-phenylenedimaleimide (pPDM). It was found that pPDM-modified S1 (pPDM-S1) binds weakly to F-actin even in the absence of nucleotides, with an affinity similar to that of unmodified S1 in the presence of ATP [63]. Therefore, pPDM-S1 is often used for studies of the weak binding of the myosin head to actin.
We applied the DSC approach described above (Fig. 6) to examine the weak binding of pPDM-S1 to F-actin stabilized by phalloidin. It was found that F-actin affects the thermal unfolding of pPDM-S1 only at very low ionic strength, when about 40% of pPDM-S1 binds weakly to F-actin, but not at higher ionic strength (200 mM KCl) preventing the interaction of pPDM-S1 with F-actin. The weak binding of pPDM-S1 to F-actin shifted the thermal transition of pPDM-S1 by about 5°C to higher temperature [64]. This actin-induced increase in thermal stability of pPDM-S1 was similar to that observed with “strong” binding of unmodified S1 to F-actin (Fig. 6).
These results show that actin-induced structural changes that are revealed by DSC in the myosin head occur not only upon strong binding but also on weak binding of the head to F-actin. This suggests that these actin-induced changes may occur before the power-stroke and play an important role in the motor function of the head. This assumption is corroborated by the DSC data demonstrating that these structural changes are strongly affected by charge changes in loop 2 [62], i.e., in the site mainly responsible for the weak binding of the myosin head to F-actin. We may speculate that since these structural changes occur in the myosin head on forming the weak binding state they must occur during the initial steps of actin-myosin interaction, and therefore they may play an important role for the transition of actin-bound myosin head from the weakly bound state to the strongly bound state.
Effects of Specific Modifications of the Myosin Head on Its Ability to Undergo the Global Structural Changes
Hence, the use of DSC represents a powerful experimental approach for probing the global structural changes that occur in the myosin head during the ATPase reaction and due to interaction with actin. We have elaborated special approaches for probing these nucleotide-induced and actin-induced structural changes in the head by measuring the changes in the thermal unfolding of the protein (see Figs. 5-7). The main goal of the following studies was to understand the mechanism of these changes, i.e., the mechanism of transmission of structural changes from the nucleotide- and actin-binding sites to the entire motor portion of the head. For this purpose, specially modified preparations of the myosin head were studied by DSC to reveal their ability to undergo global conformational changes due to interaction with F-actin and nucleotides. The most interesting modifications were those that did not directly affect the actin- and nucleotide-binding sites, but impaired the spread of conformational changes from these sites to the entire motor domain of the myosin head.
Modifications selectively preventing the global structural changes induced in the myosin head by ADP and Pi analogs. First of all, one of these modifications is the above-described pPDM cross-linking between SH-groups of Cys707 and Cys697 in the S1 molecule. The pPDM-S1 demonstrated the actin-induced structural changes, but its thermal unfolding was not affected by the addition of ADP and Pi analogs [64]. However, the pPDM-S1 was unable to form the ternary complexes with ADP and Pi analogs [65].
Another case, which is much more interesting in this respect, is modification of both “essential” SH-groups in rabbit skeletal S1 (SH1 and SH2 of residues Cys707 and Cys697, respectively) by various thiol reagents, without cross-linking between the SH-groups [66]. This modification had no effect on the actin-induced changes in the thermal unfolding of S1, but it almost fully prevented the changes induced by the formation of the ternary complexes S1-ADP-Vi, S1-ADP-AlF4-, and, to some extent, S1-ADP-BeFx. On the other hand, EPR studies on S1 spin-labeled at the SH1 group showed that modification of both SH1 and SH2 groups has no effect on the local conformational changes, which occur around the SH1 group due to formation of the S1 ternary complexes with ADP and Pi analogs [66]. Thus, the combined use of the DSC and EPR has shown that modification of both SH-groups, SH1 and SH2, does not prevent the local conformational changes induced by nucleotides around the SH1 group, but this modification strongly prevents the global nucleotide-induced structural changes of the entire S1 molecule. These results suggest that modification of SH1 (Cys707) and SH2 (Cys697) impair the spread of nucleotide-induced conformational changes from the ATPase site throughout the structure of the entire S1 molecule, thus disturbing a coupling between functionally important sites in the myosin head.
Similar effects were induced in the motor domain of the head of D. discoideum myosin II by replacing of Phe506 by glycine (mutation F506G) [67]. DSC data showed that this mutation completely prevents conformational changes that normally occur upon formation of the ternary complexes with ADP and Pi analogs, but it has almost no influence on the actin-induced changes (the actin-induced shift of the thermal transition to higher temperature, DeltaTm, was equal to 4.5°C for this mutant construct). The mutant construct displayed no motor activity in vitro [67]. It was proposed that the mutation F506G disrupts the communication between the nucleotide-binding site and the “converter” region in the motor domain of the myosin head, thus preventing transmission of structural changes from the ATPase site to the converter, which is responsible for the movement of the regulatory domain (lever arm) relative to the motor domain.
It is important to note that both the above-described modifications were in regions connecting different subdomains in the motor domain of the myosin head (i.e., in the regions connecting converter with N-terminal subdomain and with actin-binding subdomain). Probably these modifications impeded the movement of subdomains, thus preventing the work of the myosin head as the molecular motor.
Modifications selectively preventing only the actin-induced structural changes in the myosin head. It was mentioned above that charge changes in the actin-binding loop 2 do not significantly affect the nucleotide-induced structural changes in the myosin motor domain, but they affect structural changes that occur when the motor domain is strongly bound to F-actin. The actin-induced structural changes were prevented almost completely by insertions with multiple negative charges [62] or by deletion of loop 2 [68]. For example, mutant constructs M765(20/-10) (the isolated motor domain of the head of D. discoideum myosin II, M765, with 20 additional residues inserted into loop 2, including 10 negatively charged residues) [62] and M765-NL (M765 with loop 2 deleted) [68] fully retained the ability of M765 to undergo global structural changes due to ADP binding and the formation of stable ternary complexes with ADP and Pi analogs. In contrast, actin binding to these mutant constructs had no or negligible effect on their thermal unfolding (e.g., the DeltaTm value was only 0.6°C for M765-NL).
These effects can be explained easily, as loop 2 is part of the actin-binding site and, therefore, alterations in this loop affect the actin-myosin interaction and those structural changes, which occur in the myosin head due to this interaction. The DSC experiments with pPDM-S1 cited above suggested that the interaction of loop 2 with actin is mainly responsible for actin-induced structural changes in the myosin head that are expressed in a pronounced shift of the thermal transition to higher temperature. In this respect, an intriguing result has recently been obtained by DSC study on S1 cleaved by trypsin in the N-terminal region of the heavy chain, between Arg23 and Ile24 [69]. It was shown that this cleavage has no effect on the nucleotide-induced structural changes in S1, but it prevents the changes that occur when S1 is bound to F-actin.
The effect of the N-terminal cleavage of the S1 heavy chain (i.e., the absence of the shift to higher temperature of the thermal transition of modified S1 bound to F-actin [69]) cannot be explained by direct interaction between N-terminal region and loop 2 in S1 as these sites are spatially located rather far from each other in the atomic structure of S1 [24] (Fig. 4). It seems more likely that a long-distance communication pathway exists between these sites. The cleavage between Arg23 and Ile24 probably disrupts this communication pathway, thus preventing the global conformational changes in the myosin head induced by actin binding to loop 2.
In conclusion, the DSC approach makes it possible to reveal the global nucleotide-induced and actin-induced structural changes in the myosin head. This approach, in combination with other methods, allows us to investigate long-distance communication pathways between functionally important but spatially far regions in the myosin head. All these help us to understand the mechanism of functioning of the myosin head as the molecular motor.
This work was supported in part by the Russian Foundation for Basic Research (grant 03-04-48237), by the Program for the Support of the Leading Scientific Schools in Russian Federation (grant NSH-813.2003.4), by the Program “Molecular and Cell Biology” of Russian Academy of Sciences, by The Welcome Trust (grant 066115/Z/01/Z), and by INTAS (grant 03-51-4813).
REFERENCES
1.Engelhardt, V. A., and Ljubimova, M. N. (1939)
Nature, 144, 668-669.
2.Poglazov, B. F. (1965) Structure and Functions
of Contractile Proteins [in Russian], Nauka, Moscow.
3.Poglazov, B. F. (1966) Structure and Functions
of Contractile Proteins, Academic Press, N. Y.
4.Poglazov, B. F., and Levitsky, D. I. (1982)
Myosin and Biological Motility [in Russian], Nauka, Moscow.
5.Engelhardt, V. A., Ljubimova, M. N., and Meitina,
R. A. (1941) Dokl. Akad. Nauk SSSR, 30, 639-641.
6.Engelhardt, V. A., and Ljubimova, M. N. (1942)
Biokhimiya, 7, 205-231.
7.Szent-Gyorgyi, A. G. (1949) Biol. Bull.,
96, 140-161.
8.Huxley, A. F., and Niedergerke, R. (1954)
Nature, 173, 971-973.
9.Huxley, H. E., and Hanson, J. (1954) Nature,
173, 973-976.
10.Lowey, S., Slayter, H. S., Weeds, A. G., and
Baker, H. (1969) J. Mol. Biol., 42, 1-29.
11.Taylor, E. W. (1977) Biochemistry,
16, 732-740.
12.Harrington, W. F. (1979) Proc. Natl. Acad.
Sci. USA, 76, 5066-5070.
13.Poglazov, B. F., Samokhin, G. P., Klibanov, A.
M., Levitsky, D. I., Martinek, K., and Berezin, I. V. (1978)
Biochim. Biophys. Acta, 524, 245-253.
14.Poglazov, B. F. (1961) Byul. Eksp. Biol.
Med., 9, 56-59.
15.Poglazov, B. F. (1962) Biokhimiya,
27, 161-166.
16.Vorobjeva, I. A., and Poglazov, B. F. (1963)
Biofizika, 8, 427-429.
17.Pollard, T. D., and Korn, E. D. (1973) J.
Biol. Chem., 248, 4682-4690.
18.Hammer, J. A., Korn, E. D., and Paterson, B. M.
(1984) J. Biol. Chem., 259, 11157-11159.
19.Hammer, J. A. (1994) J. Muscle Res. Cell
Motil., 15, 1-10.
20.Sellers, J. R., Goodman, H. V., and Wang, F.
(1996) J. Muscle Res. Cell Motil., 17, 7-22.
21.Hodge, T., and Cope, M. J. T. V. (2000) J.
Cell Sci., 113, 3353-3354.
22.Cheney, R. E., and Mooseker, M. S. (1992)
Curr. Opin. Cell Biol., 4, 27-35.
23.Toyoshima, Y. Y., Kron, S. J., McNully, E. M.,
Niebling, K. R., Toyoshima, C., and Spudich, J. A. (1987)
Nature, 328, 536-539.
24.Rayment, I., Rypniewski, W. R., Schmidt-Base, K.,
Smith, R., Tomchick, D. R., Benning, M. M., Winkelmann, D. A.,
Wesenberg, G., and Holden, H. M. (1993) Science, 261,
50-58.
25.Spudich, J. A. (1994) Nature, 372,
515-518.
26.Uyeda, T. Q., Abramson, P. D., and Spudich, J. A.
(1996) Proc. Natl. Acad. Sci. USA, 93, 4459-4464.
27.Anson, M., Geeves, M. A., Kurzawa, S. E., and
Manstein, D. J. (1996) EMBO J., 15, 6069-6074.
28.Rief, M., Rock, R. S., Mehta, A. D., Mooseker, M.
S., Cheney, R. E., and Spudich, J. A. (2000) Proc. Natl. Acad.
Sci. USA, 97, 9482-9486.
29.Goodno, C. C. (1982) Meth. Enzymol.,
85, 116-123.
30.Phan, B. C., and Reisler, E. (1992)
Biochemistry, 31, 4787-4793.
31.Werber, M. M., Peyser, Y. M., and Muhlrad, A.
(1992) Biochemistry, 31, 7190-7197.
32.Ponomarev, M. A., Timofeev, V. P., and Levitsky,
D. I. (1995) FEBS Lett., 371, 261-263.
33.Phan, B. C., Peyser, Y. M., Reisler, E., and
Muhlrad, A. (1997) Eur. J. Biochem., 243, 636-642.
34.Fisher, A. J., Smith, C. A., Thoden, J., Smith,
R., Sutoh, K., Holden, H. M., and Rayment, I. (1995)
Biochemistry, 34, 8960-8972.
35.Smith, C. A., and Rayment, I. (1996)
Biochemistry, 35, 5404-5417.
36.Gopal, D., and Burke, M. (1996)
Biochemistry, 35, 506-512.
37.Bobkov, A. A., Khvorov, N. V., Golitsina, N. L.,
and Levitsky, D. I. (1993) FEBS Lett., 332, 64-66.
38.Bobkov, A. A., and Levitsky, D. I. (1995)
Biochemistry, 34, 9708-9713.
39.Dominguez, R., Freazon, Y., Trybus, K. M., and
Cohen, C. (1998) Cell, 94, 559-571.
40.Houdusse, A., Kalabokis, V. N., Himmel, D.,
Szent-Gyorgyi, A. G., and Cohen, C. (1999) Cell, 97,
459-470.
41.Houdusse, A., Szent-Gyorgyi, A. G., and Cohen, C.
(2000) Proc. Natl. Acad. Sci. USA, 97, 11238-11243.
42.Rayment, I., Holden, H. M., Whittaker, M., Yohn,
C. B., Lorenz, M., Holmes, K. C., and Milligan, R. A. (1993)
Science, 261, 58-65.
43.Privalov, P. L. (1982) Adv. Protein Chem.,
35, 1-104.
44.Privalov, P. L., and Potekhin, S. A. (1986)
Meth. Enzymol., 131, 4-51.
45.Khvorov, N. V., Levitsky, D. I., Bukatina, A. E.,
Shnyrov, V. L., and Poglazov, B. F. (1990) Dokl. Akad. Nauk
SSSR, 315, 745-748.
46.Levitsky, D. I., Shnyrov, V. L., Khvorov, N. V.,
Bukatina, A. E., Vedenkina, N. S., Permyakov, E. A., Nikolaeva, O. P.,
and Poglazov, B. F. (1992) Eur. J. Biochem., 209,
829-835.
47.Levitsky, D. I., Bobkov, A. A., Golitsina, N. L.,
Nikolaeva, O. P., Pavlov, D. A., and Poglazov, B. F. (1996)
Biofizika, 41, 64-72.
48.Levitsky, D. I., Nikolaeva, O. P., Orlov, V. N.,
Pavlov, D. A., Ponomarev, M. A., and Rostkova, E. V. (1998)
Biochemistry (Moscow), 63, 322-323.
49.Levitsky, D. I. (2004) Uspekhi Biol.
Khim., 44, 133-170.
50.Gopal, D., Bobkov, A. A., Schwonek, J. P.,
Sanders, C. R., Ikebe, M., Levitsky, D. I., and Burke, M. (1995)
Biochemistry, 34, 12178-12184.
51.Gopal, D., Pavlov, D. A., Levitsky, D. I., Ikebe,
M., and Burke, M. (1996) Biochemistry, 35,
10149-10157.
52.Levitsky, D. I., Ponomarev, M. A., Geeves, M. A.,
Shnyrov, V. L., and Manstein, D. J. (1998) Eur. J. Biochem.,
251, 275-280.
53.Pavlov, D. A., Sobieszek, A., and Levitsky, D. I.
(1998) Biochemistry (Moscow), 63, 952-962.
54.Pavlov, D. A., Bobkov, A. A., Nikolaeva, O. P.,
Magretova, N. N., Dedova, I. V., and Levitsky, D. I. (1995)
Biochemistry (Moscow), 60, 835-842.
55.Golitsina, N. L., Bobkov, A. A., Dedova, I. V.,
Pavlov, D. A., Nikolaeva, O. P., Orlov, V. N., and Levitsky, D. I.
(1996) J. Muscle Res. Cell Motil., 17, 475-485.
56.Golitsina, N. L., Shnyrov, V. L., and Levitsky,
D. I. (1992) FEBS Lett., 303, 255-257.
57.Levitsky, D. I., Khvorov, N. V., Shnyrov, V. L.,
Vedenkina, N. S., Permyakov, E. A., and Poglazov, B. F. (1990) FEBS
Lett., 264, 176-178.
58.Levitsky, D. I., Nikolaeva, O. P., Vedenkina, N.
S., Shnyrov, V. L., Golitsina, N. L., Khvorov, N. V., Permyakov, E. A.,
and Poglazov, B. F. (1991) Biomed. Sci., 2, 140-146.
59.Levitsky, D. I. (1994) in Soviet Sci.
Rev. - Phys.-Chem. Biol., Vol. 12, Pt. 1 (Skulachev, V. P.,
ed.) Harwood Academic Publishers, pp. 1-53.
60.Levitsky, D. I. (1991) Biokhimiya,
56, 1539-1566.
61.Zubov, E. O., and Levitsky, D. I. (2002) J.
Muscle Res. Cell Motil., 23, 15.
62.Ponomarev, M. A., Furch, M., Levitsky, D. I., and
Manstein, D. J. (2000) Biochemistry, 39, 4527-4532.
63.Chalovich, J. M., Greene, L. E., and Eisenberg,
E. (1983) Proc. Natl. Acad. Sci. USA, 80, 4909-4913.
64.Kaspieva, O. V., Nikolaeva, O. P., Orlov, V. N.,
Ponomarev, M. A., and Levitsky, D. I. (2001) FEBS Lett.,
489, 144-148.
65.Bobkov, A. A., and Reisler, E. (2000) Biophys.
J., 79, 460-467.
66.Levitsky, D. I., Shakirova, L. I., Mikhailova, V.
V., Siletskaya, E. I., and Timofeev, V. P. (2001) Abst. XXX
Eur. Muscle Conf., Pavia, Italy, p. 85.
67.Tsiavaliaris, G., Fujita-Becker, S., Batra, R.,
Levitsky, D. I., Kull, F. J., Geeves, M. A., and Manstein, D. J.
(2002) EMBO Rep., 3, 1099-1105.
68.Ponomarev, M., Furch, M., Knetsch, M., Manstein,
D., and Levitsky, D. (1999) J. Muscle Res. Cell Motil.,
20, 72.
69.Nikolaeva, O. P., Orlov, V. N., Bobkov, A. A.,
and Levitsky, D. I. (2002) Eur. J. Biochem., 269,
5678-5688.