|
REVIEW: Anti-angiogenic, Antioxidant, and Anti-carcinogenic Properties of a Novel Anthocyanin-Rich Berry Extract FormulaD. Bagchi1*, C. K. Sen2, M. Bagchi1, and M. Atalay31Department of Pharmacy Sciences, Creighton University Medical Center, 2500 California Plaza, Omaha, NE 68178, USA; fax: 402-280-1883; E-mail: debsis@creighton.edu2Laboratory of Molecular Medicine, Department of Surgery, 512 Davis Heart and Lung Research Institute, The Ohio State University Medical Center, 473 W. 12th Avenue, Columbus, OH 43210, USA; fax: 614-247-7818; E-mail: sen-1@medctr.osu.edu 3Department of Physiology, University of Kuopio, P.O. Box 1627, FIN-70211, Kuopio, Finland; fax: +358-17-163-112; E-mail: Mustafa.atalay@uku.fi * To whom correspondence should be addressed.
|
Received April 30, 2003
Edible berry anthocyanins possess a broad spectrum of therapeutic and anti-carcinogenic properties. Berries are rich in anthocyanins, compounds that provide pigmentation to fruits and serve as natural antioxidants. Anthocyanins repair and protect genomic DNA integrity. Earlier studies have shown that berry anthocyanins are beneficial in reducing age-associated oxidative stress, as well as in improving neuronal and cognitive brain function. Six berry extracts (wild blueberry, bilberry, cranberry, elderberry, raspberry seeds, and strawberry) were studied for antioxidant efficacy, cytotoxic potential, cellular uptake, and anti-angiogenic (the ability to reduce unwanted growth of blood vessels, which can lead to varicose veins and tumor formation) properties. We evaluated various combinations of edible berry extracts and developed a synergistic formula, OptiBerry IH141, which exhibited high ORAC (Oxygen-Radical Absorbing Capacity) value, low cytotoxicity, and superior anti-angiogenic properties compared to the other combinations tested. Anti-angiogenic approaches to treat cancer represent a priority area in vascular tumor biology. OptiBerry significantly inhibited both H2O2- and TNF-alpha-induced VEGF (Vascular Endothelial Growth Factor) expression by human keratinocytes. VEGF is a key regulator of tumor angiogenesis. Matrigel assay using human microvascular endothelial cells showed that OptiBerry impaired angiogenesis. In an in vivo model of angiogenesis, OptiBerry significantly inhibited basal MCP-1 and inducible NF-kappaB transcriptions. Endothelioma cells pretreated with OptiBerry showed a diminished ability to form hemangioma and markedly decreased tumor growth by more than 50%. In essence, these studies highlight the novel anti-angiogenic, antioxidant, and anti-carcinogenic potential of a novel anthocyanin-rich berry extract formula, OptiBerry.
KEY WORDS: berry anthocyanins, antioxidants, angiogenesis, keratinocytes, hemangioma
During the past two decades, a growing number of studies have investigated the diverse health benefits and protective effects of anthocyanins present in various fruits and vegetables. Anthocyanins are common components of fruits and vegetables, in particular edible berries, which provide pigmentation (color) and serve as natural antioxidants [1-5]. Principal therapeutic benefits attributable to anthocyanins include antioxidant protection and maintenance of DNA integrity. Anthocyanins also serve as anti-inflammatory and anti-mutagenic agents and provide cardioprotection by maintaining vascular permeability. Studies have shown that supplementations with berries rich in anthocyanins were effective in reducing oxidative stress associated with aging, and were beneficial in reversing age-related neuronal and behavioral changes. Supplementation with anthocyanins for 6-8 months retarded age-related declines in neuronal and cognitive function by improving antioxidant status [6-8].
Here we represent an overview of some novel properties of edible berry anthocyanins with emphasis on the formulation of a new berry extract product suited for antioxidant and anti-angiogenic functions.
FREE RADICALS, ANTIOXIDANTS, AND ANTHOCYANINS
Free radicals oxidatively damage lipids and proteins and compromise genomic DNA integrity. They are widely recognized as the root cause of numerous degenerative diseases including cancer [9]. Antioxidants function as inhibitors at both initiation and promotion/propagation/transformation stages of tumor promotion/carcinogenesis, and protect cells against oxidative damage. Antioxidants are potent scavengers of free radicals and serve as inhibitors of neoplastic processes [10]. The consumption of edible plants, fruits, and vegetables has been demonstrated to prevent the occurrence of a number of diseases in humans and animals. Vegetables, fruits, and their seeds are rich sources of vitamins C, E, and beta-carotene, proanthocyanidins, anthocyanins, and/or protease inhibitors, and also compounds that might protect the organism against free radical induced injury and diseases [11, 12].
A variety of edible berry extracts have been demonstrated to exhibit a broad spectrum of benefits including cardiovascular, neurological, urinary tract, and ocular protection as well as antioxidant, anti-diabetic, skin health, and anti-aging properties. In the recent past, an increasing number of studies have investigated the diverse protective effects of anthocyanins present in various fruits and vegetables [13, 14]. Anthocyanins are common components of fruits, in particular, berries. Anthocyanins protect DNA integrity and bolster tissue antioxidant levels. Studies have shown that dietary supplementation with berries rich in anthocyanins were effective in reducing oxidative stress [15].
CHEMISTRY OF ANTHOCYANINS
Anthocyanins differ from other natural flavonoids in the range of colors that can be derived from them and by their ability to form resonance structures by changes in pH [16]. The purported health benefits of red wine are thought to be derived from their phenolic content, principally flavonoids, which have demonstrated powerful antioxidant properties against low density lipoprotein oxidation [17]. The general antioxidant effect of red wines correlates with their total phenolic content [18]. Anthocyanins are the main class of flavonoids in red wines. They are responsible for the color and powerful antioxidant properties of red wine [19]. The 3-glucoside anthocyanins--delphinidin, cyanidin, petunidin, and malvidin--are present in red wines, but malvidin 3-glucoside, malvidin 3-glucoside acetate, and malvidin 3-glucoside coumarate are the most abundant. Anthocyanins exist in an aqueous phase in a mixture of four molecular species. Their relative color depends upon pH. At pH 1-3 the flavylium cation is red colored, at pH 5 the colorless carbinol pseudo base (pb) is generated, and at pH 7-8 the blue purple quinoidal base (qb) is formed. The high levels of anthocyanins in berries contribute to their powerful antioxidant activity [16].
COMBINATION OF BERRY EXTRACTS
A novel combination of berry extracts was developed, which demonstrated the distinct features and characteristics of each individual berry extract and exhibited the optimal antioxidant potency and minimum cytotoxicity [2, 20]. In this study, ORAC (Oxygen-Radical Absorbing Capacity) assay was used as a marker of antioxidant potential and lactate dehydrogenase (LDH) activity was used as a marker to determine cytotoxicity [2]. Six berry extracts including wild blueberry, bilberry, cranberry, elderberry, raspberry seed, and strawberry were examined. Twenty different combinations of these six berry extracts were evaluated for their antioxidant efficacy and cytotoxicity, and compared with a known, powerful grape seed proanthocyanidin extract (GSPE). ORAC assays were done based on a previous study by Cao and coworkers [21].
Wild blueberry and bilberry extracts exhibited the highest ORAC values compared to the other samples, while strawberry powder exhibited a significantly higher ORAC value compared to cranberry, elderberry, and raspberry seed [2]. Cranberry exhibited a marginally higher ORAC value compared to elderberry, while elderberry exhibited a marginally higher ORAC value compared to raspberry seed [2]. Based on these initial ORAC values and on a mathematical model, a combination of edible berry anthocyanins, OptiBerry IH141, was developed [2, 20]. Wild blueberry, bilberry, and OptiBerry IH141 exhibited higher ORAC values compared to GSPE, which exhibited superior antioxidant properties compared to vitamin C, E, and beta-carotene [22].
Cytotoxicity was assessed by LDH assay. HaCaT keratinocytes were seeded at 0.15*106 cells per well (1 ml to 12-well plates). After 24 h of growth the medium was changed to serum-free RPMI. Berry extracts (50 µg/ml) or GSPE (25 µg/ml) were added to the cells. After 24 h media was collected for LDH assay, which was performed using the in vitro toxicology assay kit obtained from Sigma (USA). OptiBerry and other berry extracts exhibited no statistically significant cytotoxicity compared to the control sample, while GSPE exhibited approximately 1.6-fold higher LDH leakage compared to the control sample [20].
ANTI-ANGIOGENIC PROPERTIES OF EDIBLE BERRIES
Angiogenesis is a term used to describe formation of new blood vessels. Angiogenesis is unwanted in situations including varicose veins and tumor formation. This is a key event that feeds tumor growth and cancer metastases [2]. Anti-angiogenic approaches to prevent and treat cancer represent a priority area in investigative tumor biology [23, 24]. Vascular endothelial growth factor (VEGF) plays a crucial role for the vascularization of tumors [2]. The vasculature in adult skin remains normally quiescent. However, skin retains the capacity for brisk initiation of angiogenesis during inflammatory skin diseases such as psoriasis and skin cancers [25]. The effects of multiple berry extracts on inducible VEGF expression by human HaCaT keratinocytes were evaluated [2]. Six individual berry extracts (wild blueberry, bilberry, cranberry, elderberry, raspberry seed, and strawberry) and OptiBerry were investigated. These samples demonstrated significant inhibition of both H2O2- as well as TNF-alpha_.Tumor Necrosis Factor-alpha)-induced VEGF expression by human keratinocytes. OptiBerry exhibited the greatest effect in inhibiting H2O2-induced VEGF expression [2]. Table 1 demonstrates the inhibitory effects of wild blueberry, bilberry, raspberry seed, strawberry, OptiBerry, and GSPE against H2O2-induced expression of VEGF. The berries demonstrated significant inhibitory effects, while OptiBerry exhibited the maximum inhibition. GSPE exhibited no inhibitory effect.
Table 1. OptiBerry and other berry samples
inhibited H2O2-induced expression of VEGF
(vascular endothelial growth factor)
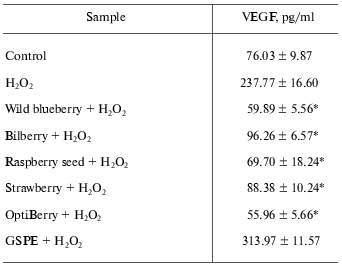
Note: HaCaT cells were seeded at density 0.45*106/well (3
ml). After 24 h, growth medium was changed to serum-free RPMI and berry
samples (50 µg/ml) or GSPE (25 µg/ml) were added.
After 12 h, cells were challenged with H2O2
(150 µM). After 12 h of activation with
H2O2, medium was collected for ELISA. Data
present mean ± SD of three experiments; * p <
0.05 (higher in response to H2O2 treatment); *
p < 0.05 (lower compared to H2O2
treated cells).
Table 2 provides an overview on the inhibitory effects of wild blueberry, bilberry, raspberry seed, strawberry, OptiBerry, and GSPE on TNF-alpha-induced expression of VEGF. Wild blueberry exhibited the maximum inhibitory effect, while no significant difference was observed among raspberry seed, strawberry, and OptiBerry. GSPE exhibited no inhibitory effect.
Table 2. OptiBerry and other berry samples
inhibited TNF-alpha-induced expression of VEGF
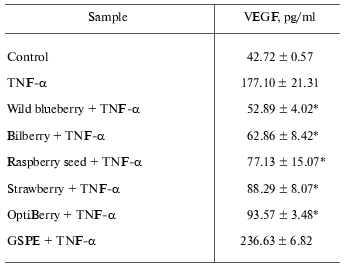
Note: HaCaT cells were seeded at density 0.45*106/well (3
ml). After 24 h, growth medium was changed to serum-free RPMI and
berry samples (50 µg/ml) or GSPE (25 µg/ml) were
added. After 12 h, cells were challenged with TNF-alpha (25
ng/ml). After 12 h of activation with TNF-alpha, medium was
collected for ELISA. Data present mean ± SD of three
experiments; * p < 0.05 (higher in response to
TNF-alpha treatment); * p < 0.05 (lower compared to
TNF-alpha treated cells).
Antioxidants such as GSPE, with comparable ORAC, or alpha-tocopherol did not influence inducible VEGF expression suggesting that the observed effect of berry extracts was not dependent on their antioxidant property alone [2]. On the contrary, pure flavonoids such as ferrulic acid, catechin, and rutin shared the ability to suppress oxidant-inducible VEGF expression.
Thus, it was evident that the flavonoid constituents of the berry extracts may have been responsible for the observed effect on inducible VEGF expression and release [2]. Table 3 demonstrates that alpha-tocopherol provides no inhibitory effect on H2O2-induced expression of VEGF, while pure flavonoids such as ferrulic acid, catechin, and rutin exhibited inhibitory effects on H2O2-induced expression of VEGF.
Table 3. Effect of alpha-tocopherol
and pure flavonoids on H2O2-induced expression of
VEGF
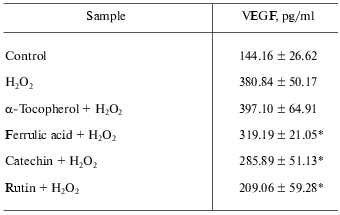
Note: HaCaT cells were seeded at density 0.45*106/well (3
ml). After 24 h, growth medium was changed to serum-free RPMI and
either pure flavonoids (200 nM ferrulic acid; 100 nM catechin;
1 µM rutin) at concentrations observed in berry samples [2] or alpha-tocopherol (10 µM, as a
reference antioxidant) was added. After 12 h, cells were
challenged with H2O2 (150 µM). After
12 h of activation with H2O2, medium was
collected for ELISA. Data present mean ± SD of three
experiments; * p < 0.05 (higher in response to
H2O2 treatment); * p < 0.05 (lower
compared to H2O2 treated cells).
In vitro MATRIGEL ASSAY DEMONSTRATING ANGIOGENESIS
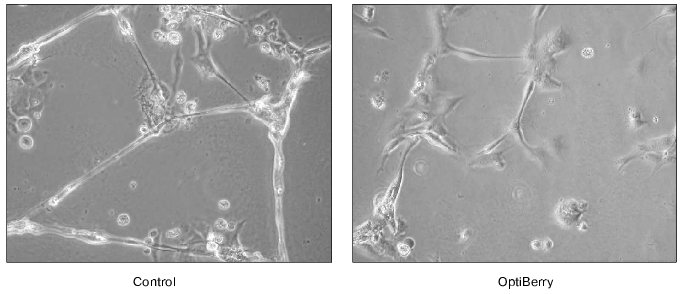
The in vitro matrigel assay represents a highly reliable approach to study angiogenesis. This method is based on the differentiation of endothelial cells to form capillary structures on a basement membrane matrix. Matrigel is a matrix of a mouse basement membrane neoplasm, which represents a complex mixture of basement membrane proteins including type IV collagen, entactin/nitrogen, proteoheparan sulfate, and other growth factors. Matrigel assays induce endothelial cells to differentiate as shown by morphological changes and by the reduction in proliferation, which offers a convenient model to study biochemical changes associated with angiogenesis [26]. Human dermal microvascular endothelial cells and an in vitro angiogenesis kit (Chemicon International, Inc., USA) were used in this assay. Briefly, ECMatrix is a solid gel of basement proteins prepared from the Engelbreth-Holm-Swarm (EHS) mouse tumor. The 100 µl of diluted ECMatrix (10×) solution was transferred to each well of a 96-well tissue culture plate and incubated at 37°C for at least an hour to allow the matrix solution to solidify. Human dermal microvascular endothelial cells were harvested in the presence and absence of berry extracts. Cells (5000 cells/well) were added on top of the solidified matrix solution and maintained in an incubator at 37°C overnight. Endothelial tube formation was observed and digitally photographed under an inverted light microscope at 20× magnification. Treatment of the human dermal microvascular endothelial cells with OptiBerry impaired angiogenesis [2] (Fig. 1).
Fig. 1. Anti-angiogenic property of OptiBerry in vitro. An in vitro model of angiogenesis was used employing Matrigel and human dermal microvascular endothelial cells. Human dermal microvascular endothelial cells (0.5*105 cells/well) were seeded onto 4-well plates precoated with Matrigel. After 48 h of seeding, OptiBerry sample was added (50 µg/ml). Endothelial tube formation is observed and digitally photographed under an inverted light microscope at 20-100× (20× shown) magnification. Representative of three experiments.
ANTI-ANGIOGENIC PROPERTY OF OPTIBERRY IN A MODEL OF HEMANGIOMA
Hemangiomas are the most common infancy tumors, which occur in approximately 1 : 100 normal newborns, however, in premature infants weighing less than 1000 g the incidence rises to 1 : 5 live births [27]. The hemangioma is characterized by rapid growth during the first year of life (proliferative phase) followed by a decline in growth (involutional phase) over the next 5-6 years, with complete regression of the lesion in 90% of affected individuals by age 9 (the involuted phase). Hemangiomas arise from clonal expansion of a single endothelial cell precursor [28]. Approximately 5% of hemangiomas cause serious tissue damage, while 1-2% of all hemangiomas are life threatening. Proliferating hemangiomas are highly angiogenic and, thus, experimentally, hemangiomas represent a powerful model to study in vivo angiogenesis.
The presence of macrophages is associated with proliferating hemangiomas. The CC chemokine, MCP-1 (monocyte chemotactic protein-1), has been shown to be responsible for recruiting macrophages to infection or inflammation sites. MCP-1 is acknowledged as a major accessory facilitating angiogenesis and a direct role of MCP-1 on angiogenesis has been recently demonstrated. Thus, antagonists to MCP-1 are considered to be anti-angiogenic [29].
The EOMA cell line has also been well characterized in the literature with regard to its derivation, expression of endothelial cell markers, production of endostatin, and response to angiostatin. In our recent study, the endothelioma (EOMA) cell line derived from the spontaneously arising hemangioma was used [30]. Following 24 h of seeding of EOMA cells, the culture medium was replaced with fresh medium and the cells were treated with OptiBerry for 12 or 24 h. High baseline luciferase activity suggests elevated levels of basal MCP-1 transcription in these EOMA cells. Cells were activated with TNF-alpha (400 IU/ml) for 12 h. MCP-1 levels in cell-free culture media were measured using a commercially available ELISA kit (R & D Systems, USA) according to the manufacturer's instructions. Pretreatment of the EOMA cells with OptiBerry significantly inhibited basal MCP-1 transcription in EOMA cells. TNF-alpha potently induced NF-kappaB (Nuclear Factor kappaB) transcription. Inducible NF-kappaB activity was also significantly lower in EOMA cells pretreated with OptiBerry.
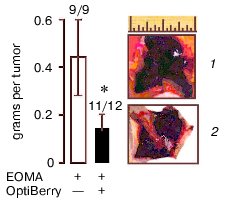
Eight-week-old 129P3/J mice (Jackson Laboratories, USA) received inhalation anesthesia and were injected subcutaneously with 100 µl of EOMA cell suspension in phosphate buffered saline for a total dose of 5*106 cells. All animal protocols were approved by the Animal Institutional Laboratory Animal Care and Use Committee (ILACUC). The tumor was harvested one week after the injection. Injection of the EOMA cells pretreated with OptiBerry did not result in hemangioma formation in all mice. Approximately, 100% of the control and 92% of the OptiBerry group tested positive for the presence of hemangioma. Although the OptiBerry treated group did test positive for the presence of hemangioma, the average mass of such tumor growth was below 50% compared to the untreated control group (Fig. 2, see color insert in this issue). Histological analysis demonstrated markedly decreased infiltration of macrophages in hemangioma of treated mice compared to placebo-treated controls.
Different classes of anti-angiogenic and anti-carcinogenic plant products include flavonoids such as silymarin, green tea catechins and polyphenols, selected antioxidants, sulfated carbohydrates, and triterpenoids [31]. Flavonoid antioxidants have been demonstrated to exhibit anti-cancer effects against several epithelial cancers [32] as well as against solid tumors [33]. The underlying mechanistic aspects of the anti-tumor activity by these structurally diverse compounds are not entirely clear yet. Several different mechanisms have been suggested. Edible berry anthocyanin-rich extracts have been shown to inhibit cellular transformation and our study exhibited the potent inhibitory effect on inducible VEGF expression. Our observation that GSPE possessing high antioxidant capacity failed to inhibit inducible VEGF expression leads us to believe that an antioxidant property alone may not account for the total observed effect. Our recent in vivo study demonstrated that OptiBerry significantly inhibits basal MCP-1 and inducible NF-kappaB transcriptions and that endothelioma cells pretreated with OptiBerry showed a diminished ability to form hemangioma. Histological analysis exhibited markedly decreased infiltration of macrophages in hemangioma of treated mice compared to the control animals.Fig. 2. Hemangioma mass, incidence, and appearance. Data collected one week after subcutaneous injection of OptiBerry-treated (2) or untreated (control) EOMA cells (1) to 129P3/J mice. Data present mean ± SD of three experiments; * lower mass compared to control group (p < 0.01). Bar graph represents the average mass of hemangioma. Scale, 1 inch.
In conclusion, the novel OptiBerry formulation exhibits high ORAC value, low cytotoxic potential, and efficient cellular uptake by endothelial cells [2]. A second set of in vitro studies exhibited the potent inhibitory effect of edible berries against oxidant-induced VEGF expression. OptiBerry exhibited the most potent inhibitory effect on H2O2-induced VEGF expression compared to the other berry extracts. Matrigel assay, an in vitro angiogenesis assay, was conducted on human dermal microvascular endothelial cells and OptiBerry significantly impaired angiogenesis. Finally, in an in vivo model, OptiBerry significantly inhibited basal MCP-1 and NF- kappaB transcriptions and markedly diminished in vivo angiogenesis.
REFERENCES
1.Francis, E. L. (1989) Crit. Rev. Food Sci.
Nutr., 28, 273-314.
2.Roy, S., Khanna, S., Alessio, H. M., Vider, J.,
Bagchi, D., Bagchi, M., and Sen, C. K. (2002) Free Rad. Res.,
36, 1023-1031.
3.Xue, H., Aziz, R. M., Sun, N., Cassady, J. M.,
Kamendulis, L. M., Xu, Y., Stoner, G. D., and Klaunig, J. E.
(2001) Carcinogenesis, 22, 831-833.
4.McCarty, M. F. (2001) Med. Hypotheses,
56, 137-154.
5.Kresty, L. A., Morse, M. A., Morgan, C., Carlton,
P. S., Lu, J., Gupta, A., Blackwood, M., and Stoner, G. D. (2001)
Cancer Res., 61, 6112-6119.
6.Ofek, I., Goldhar, J., Zafriri, D., Lis, H., Adar,
R., and Sharon, N. (1991) N. Engl. J. Med., 324,
1599.
7.Willett, W. C. (1995) Environ. Health
Perspect., 103, 165-170.
8.Harborne, J. B., and Williams, C. A. (2001) Nat.
Prod. Rep., 18, 310-333.
9.Aruoma, O. I. (1994) Food Chem. Toxicol.,
32, 671-683.
10.Bagchi, D., Garg, A., Krohns, R. L., Bagchi, M.,
Tran, M. X., and Stohs, S. J. (1997) Res. Commun. Mol. Pathol.
Pharmacol., 95, 179-189.
11.Tapiero, H., Tew, K. D., Ba, G. N., and Mathe, G.
(2002) Biomed. Pharmacother., 56, 200-207.
12.Harborne, J. B., and Williams, C. A. (2000)
Phytochemistry, 55, 481-504.
13.Amouretti, M. (1972) Therapeutique,
48, 579-581.
14.Bettini, V., Fiori, A., Martino, R., Mayellaro,
F., and Ton, P. (1985) Fitoterapia, 56, 67-72.
15.Narayan, M. S., Naidu, K. A., Ravishankar, G. A.,
Srinivas, L., and Venkataraman, L. V. (1999) Prostaglandins, Leukot.
Essent. Fatty Acids, 60, 1-4.
16.Camire, M. E. (2000) in Herbs, Botanicals
Teas (Mazza, G., and Oomah, B. D., eds.) Lancaster, PA, pp.
289-319.
17.Cao, G., Russell, R. M., Lischner, N., and Prior,
R. L. (1998) J. Nutr., 128, 2383-2390.
18.Waterhouse, A. L. (2002) Ann. N. Y. Acad.
Sci., 957, 21-36.
19.Brouillard, R., George, F., and Fougerousse, A.
(1997) Biofactors, 6, 403-410.
20.Yasmin, T., Sen, C. K., Hazra, S., Bagchi, M.,
Bagchi, D., and Stohs, S. J. (2003) Res. Commun. Pharmacol.
Toxicol., in press.
21.Cao, G., Alessio, H. M., and Cutler, R. G. (1993)
Free Rad. Biol. Med., 14, 303-311.
22.Bagchi, D., Bagchi, M., Stohs, S. J., Das, D. K.,
Ray, S. D., Kuszynski, C. A., Joshi, S. S., and Preuss, H. G. (2000)
Toxicology, 148, 187-197.
23.Giavazzi, R., and Taraboletti, G. (1999)
Forum, 9, 261-272.
24.Griffioen, A. W., and Molema, G. (2000)
Pharmacol. Rev., 52, 237-268.
25.Detmar, D. (2000) J. Dermatol. Sci.,
24, S78-S84.
26.Ponce, M. L., Nomizu, M., and Kleinman, H. K.
(2001) FASEB J., 15, 1389-1397.
27.Folkman, J., Mulliken, J., and Ezekowitz, R.
(1997) in Surgery of Infants and Children: Scientific Principles and
Practices (Oldham, K., ed.) Lippincott-Raven, PA, pp. 569-584.
28.Boye, E., Yu, Y., Paranya, G., Mulliken, J.,
Olsen, B., and Bischoff, J. (2001) J. Clin. Invest., 107,
745-752.
29.Salcedo, R., Ponce, M. L., Young, H. A.,
Wasserman, K., Ward, J. M., Kleinman, H. K., Oppenheim, J. J., and
Murphy, W. J. (2000) Blood, 96, 34-40.
30.Atalay, M., Gordillo, G., Roy, S., Rovin, B.,
Bagchi, D., Bagchi, M., and Sen, C. K. (2003) FEBS Lett.,
544, 252-257.
31.Paper, D. H. (1998) Planta Med.,
64, 686-695.
32.Jiang, C., Agarwal, R., and Lu, J. (2000)
Biochem. Biophys. Res. Commun., 276, 371-378.
33.Fotsis, T., Pepper, M. S., Aktas, E., Breit, S.,
Rasku, S., Adlercreutz, H., Wahala, K., Montesano, R., and Schweigerer,
L. (1997) Cancer Res., 57, 2916-2921.