Detection of Annexin IV in Bovine Retinal Rods
E. Yu. Zernii, N. K. Tikhomirova, P. P. Philippov*, and I. I. Senin
Laboratory of Visual Reception, Department of Cell Signaling, Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119992 Russia; fax: (095) 939-0978; E-mail: ppph@belozersky.msu.ru* To whom correspondence should be addressed.
Received July 23, 2002; Revision received September 6, 2002
The fraction of proteins capable of binding to photoreceptor membranes in a Ca2+-dependent manner was isolated from bovine rod outer segments. One of these proteins with apparent molecular mass of 32 kD (p32) was purified to homogeneity and identified as annexin IV (endonexin) by MALDI-TOF mass-spectrometry. In immunoblot, annexin IV purified from bovine rod outer segments cross-reacted with antibodies against annexin IV from bovine liver. This is the first detection of annexin IV in vertebrate retina.
KEY WORDS: annexin IV, Ca2+-binding proteins, retinal rods, retina
The components of molecular mechanisms providing transmission, switching, and regulation of light transmission in vertebrate photoreceptor cells--rods and cones--are concentrated in their outer segments. Of photoreceptor cells of these two types, retinal rods are studied better, and below we shall consider the processes in these cells, precisely in rod outer segments (ROS). Calcium ions play a key role in regulation of the processes participating in light switching and light adaptation of photoreceptor cells. Of these processes, phosphorylation of photoexcited rhodopsin catalyzed by rhodopsin kinase, synthesis of photoreceptor secondary messenger cGMP by the action of guanylate cyclase, and conductance of the cation channels localized in plasmatic membrane of photoreceptor cells are the best-studied. The effects of Ca2+ on the mentioned processes are mediated by Ca2+-binding proteins, whose functions in the photoreceptor cells have been investigated: for example, recoverin functioning as calcium sensor of rhodopsin kinase, three proteins called GCAP (guanylate cyclase activating protein), and S100B protein which modulate guanylate cyclase in a Ca2+-dependent manner and calmodulin regulating conductance of the cation channels [1].
Earlier several proteins with apparent molecular masses ~20, 21 (minor band), 26, 32, 70, and 80 kD binding to the photoreceptor membranes in a Ca2+-dependent manner were detected in bovine ROS [2]. It is possible that protein bands with apparent masses ~20, 21, 26, and 70 kD correspond to calmodulin [3], guanylate cyclase 1 activator (GCAP 1) [4], recoverin [1], and rhodopsin kinase [1], respectively. The latter does not bind calcium ions by itself, but in their presence forms a complex with recoverin which allows its interaction with the membrane in a Ca2+-dependent manner [1, 5, 6]. The above mentioned four proteins are now well-studied. There is no information about the nature of the protein with molecular mass 80 kD. As for the protein with molecular mass 32 kD, we suggested that it can be one of the annexins, Ca2+-binding proteins, because molecular masses of most annexins vary from 32 to 38 kD [7].
Considering that the functional importance of Ca2+-binding proteins detected in the photoreceptor cell was demonstrated earlier and that data on the presence of annexins in the photoreceptor cell were absent from the literature, we isolated p32 from bovine ROS and purified it to homogeneity. Using mass-spectrometry, we identified p32 as annexin IV (endonexin). This is the first detection of annexin IV in the vertebrate retina.
MATERIALS AND METHODS
ROS preparations were obtained from bovine retina frozen at -70°C as described in [8] and stored frozen at -70°C.
Protein concentration was determined spectrophotometrically according to Bradford [9] but with some modifications.
Electrophoresis in polyacrylamide gel in the presence of SDS (SDS-PAGE) was performed according to Laemmli [10] in 12 and 5% separating and concentrating gels, respectively. A commercial protein mixture from Bio-Rad (USA) was used as molecular mass markers. After electrophoresis the proteins were stained with Coomassie Brilliant Blue (Servablue G) or silver nitrate in the presence of 2.5% Na2CO3 containing 0.02% formaldehyde [11].
Purification of p32 from bovine ROS included the following procedures performed at 4°C and based on the method described earlier [2]. Frozen ROS preparation was suspended in 20 mM Tris-HCl buffer, pH 7.5, containing 5 mM MgCl2 (buffer A), with addition of 1 mM mercaptoethanol and 0.1 mM phenylmethylsulfonyl fluoride, and also 2 mM CaCl2 and 100 mM NaCl. Then the suspension was centrifuged (100,000g, 60 min) and the membrane pellet was washed from the soluble proteins with the same buffer, then with buffer A with addition of 0.5 mM CaCl2 and 100 µM GTP and twice with buffer A containing only 0.5 mM CaCl2. In each case the membranes after washing were collected by centrifugation at 100,000g for 60 min. Then proteins bound to the photoreceptor membranes in the presence of Ca2+ were extracted with buffer A containing 2 mM EGTA, and the extract was dialyzed against 25 mM Hepes, pH 7.3 (buffer B) overnight. The resulting solution was applied onto a MonoQ HP 5/5 column equilibrated with buffer B and chromatographed using a FPLC chromatograph from Amersham Pharmacia Biotech (Great Britain) at the elution rate 1 ml/min. First proteins were eluted with buffer B, then with NaCl gradient (0-1 M) in the same buffer. Most of the p32 was found in fractions eluted at NaCl concentration 195-200 mM.
Study of Ca2+-dependent interaction of p32 with photoreceptor membranes was performed as follows. To remove soluble proteins, photoreceptor membranes were incubated in 5 M urea, then the suspension was centrifuged (100,000g, 60 min) and the membrane pellet was washed thrice with 15 mM Hepes, pH 7.3. After each washing the membranes were collected by centrifugation at 100,000g for 60 min. The membranes thus obtained were used for study of their binding to p32. For this, p32 (4 µg) was added to two samples (total volume 50 µl each) containing a suspension of photoreceptor membranes washed with urea (rhodopsin mass in photoreceptor membranes, 10 µg) in 15 mM Hepes, pH 7.3. One sample contained 2 mM CaCl2 (“+Ca2+”-sample) and another contained 2 mM EGTA (“-Ca2+”-sample). After 5-min incubation both samples were centrifuged using a Aerofuge ultracentrifuge from Beckman (USA) at 150,000g for 15 min and the supernatants were analyzed by SDS-PAGE. The pellets were resuspended in the initial buffer which contained 2 mM EGTA in both cases, centrifuged under the same conditions, and the supernatants were collected and analyzed by SDS-PAGE.
Annexin IV from bovine liver was obtained according to the procedure described in [12] which included Ca2+-dependent protein extraction from liver homogenate with subsequent chromatography on phenyl-Sepharose and then on Q-Sepharose. The protein preparation was stored at -20°C.
Polyclonal (monospecific) antibodies against annexin IV from bovine liver were obtained by rabbit immunization according the standard procedure [13]. Immune serum was then chromatographed on a column with immobilized annexin IV purified from bovine liver (protein concentrations and experimental conditions were the same as in the procedure of obtaining antibodies against transketolase [13]). Polyclonal (monospecific) antibodies thus obtained were concentrated by centrifugation on Millipore-3000 NMWL membranes from Millipore (USA) at 2500g for 40 min and stored at -20°C.
Immunoblotting of ROS extractwas performed according to a standard procedure [14] but with our modifications. ROS were isolated from 50 bovine retinas, homogenized in buffer A containing 5 mM EGTA, 100 mM NaCl, 0.5 mM dithiothreitol, and 0.2 mM phenylmethylsulfonyl fluoride, and the homogenate was centrifuged (29,000g, 60 min, 4°C) and the supernatant was analyzed by immunoblotting. For this, the supernatant was subject to SDS-PAGE according to Laemmli [10] at concentrations of separating and concentrating gels equal to 12 and 5%, respectively. After electrophoresis the proteins were placed on nitrocellulose membranes in Tris-glycine buffer, pH 8.3, containing 20% C2H5OH at 300 mA for 1 h, membranes were incubated for 1.5 h in 20 mM Tris-HCl buffer, pH 7.5, containing 300 mM NaCl and 0.1% Tween-20 (buffer B) with addition of 10% dry milk, and then overnight in solution of initial antibodies (dilutions are given in the Fig. 4 legend) in buffer B. Then membranes were washed thrice with buffer B and incubated during 1.5 h in the presence of secondary antibodies against rabbit immunoglobulins conjugated with horseradish peroxidase from Amersham Pharmacia Biotech in buffer B (dilution 1 : 1000). After this the membranes were washed thrice with buffer B and incubated in 0.5 M Tris-HCl, pH 7.2, containing 0.1% H2O2 and 0.2% 3,3´-diaminobenzidine to color appearance.
Identification of p32 was performed as follows. The purified p32 preparation was subject to SDS-PAGE and the obtained gel was stained with silver nitrate [11]. The p32 band was cut from the gel and sequentially washed with water, a mixture of NaHCO3 and acetonitrile (1 : 1 w/w), pure acetonitrile, and 0.1 M NH4HCO3. Then the gel was incubated in acetonitrile for 15 min, dried under vacuum, resuspended in buffer containing 0.1 M NH4HCO3 and 10 mM dithiothreitol (all the above mentioned procedures were performed at room temperature), incubated at 56°C for 45 min, and then cooled to room temperature. Then the gel was incubated in buffer containing 0.1 M NH4HCO3 and 55 mM iodoacetamide for 30 min at room temperature, washed with 0.1 M NH4HCO3 and acetonitrile, and dried under vacuum. The dried gel was resuspended in buffer containing 50 mM NaHCO3, 5 mM CaCl2, and trypsin (taking 12.5 ng per µl of the mixture) and incubated in an ice bath for 45 min. The suspension was centrifuged at 15,600g for 5 min, the pellet was resuspended in the same buffer but without trypsin, then the reaction mixture was incubated for 12 h at 37°C. After incubation peptides present in the reaction mixture were sequentially eluted from the gel with 25 mM NH4HCO3, 5% formic acid, and acetonitrile. The extracts were pooled and evaporated to dryness under vacuum; this precipitate was dissolved in 5% formic acid and used for mass-spectrometry. Peptide fragments of p32 were analyzed by mass-spectrometry using a Matrix-Assisted Laser Desorption Ionization-TOF Voyager-DE Mass Spectrometer from PerSeptive Biosystems (USA) with DHB matrix. Subsequent identification of p32 was performed by fitting the mass-spectrometric data on the p32 peptide fragments to data from the SWISS-PROT/TrEMBL amino acid sequence database using the ExPASy PeptIdent program.
RESULTS AND DISCUSSION
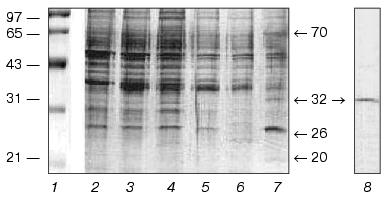
As a result of the five-fold sequential extraction of photoreceptor membranes with Ca2+-containing buffer, many proteins initially bound to membranes transfer to the solution (Fig. 1, lanes 2-6). Subsequent washing of these membranes in the presence of EGTA results in appearance of four new proteins with apparent molecular masses ~20, 26, 32, and 70 kD in the eluate (Fig. 1, lane 7). (The band of one more protein with apparent molecular mass ~80 kD as discussed in [2] was in our case masked by other relatively high-molecular-weight proteins.)
Based on ability of p32 to interact with photoreceptor membranes in a Ca2+-dependent manner and also on the fact that molecular masses of most annexins vary from 32 to 38 kD, we suggested that the protein with molecular mass 32 kD (p32) can be one of the annexins. Since earlier nothing was known about the presence of these Ca2+-binding proteins in photoreceptor cells, we purified p32 for its subsequent identification.Fig. 1. Purification of p32 from bovine ROS: 1) protein markers with their molecular masses (kD) at the left; 2-6) fractions obtained by five sequential extractions of photoreceptor membranes with Ca2+-containing buffer; 7) subsequent extraction with EGTA-containing buffer; 8) purified p32 preparation after FPLC on a MonoQ column. Apparent molecular masses of proteins (kD) eluted from membranes with EGTA-containing buffer are shown by arrows. In this and subsequent experiments, proteins were separated by electrophoresis in SDS-containing 12.5% polyacrylamide gel (experimental conditions are described in “Materials and Methods”).
Of the considered methods for p32 purification, FPLC on a MonoQ column from Amersham Pharmacia Biotech appeared to be the simplest and most efficient. The protein fraction obtained by extraction with EGTA-containing buffer of photoreceptor membranes which were washed before with Ca2+-containing buffer (Fig. 1, lane 7) was the initial material for this method. The proteins applied on a column were then eluted with a linear NaCl gradient (0-1 M) in Hepes, pH 7.5, most part of p32 being found in the fraction eluted at NaCl concentrations 195-200 mM. SDS-PAGE demonstrated homogeneity of the p32 preparation (Fig. 1, lane 8).
As shown above, p32 in the composition in the initial ROS extract is able to interact with photoreceptor membranes in a Ca2+-dependent manner. However, it cannot be excluded that p32 by itself is not sensitive to calcium ions and this property is in fact caused by the presence of a soluble Ca2+-binding protein in the system; the latter may be involved in binding p32 to membranes in a Ca2+-dependent manner.
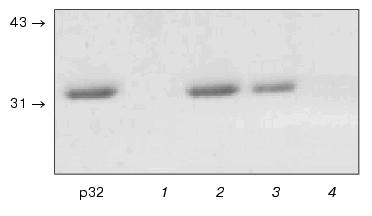
To check this possibility, we performed the following experiment: p32 was incubated in suspension of photoreceptor membranes preliminarily washed from soluble proteins with 5 M urea. Incubation was performed in parallel in samples containing 2 mM CaCl2 or 2 mM EGTA (subsequently referred to as “+Ca2+” and “-Ca2+” samples). The samples were centrifuged and the supernatants were analyzed by SDS-PAGE. As shown in Fig. 2, p32 is absent from the supernatant corresponding to the “+Ca2+” sample (lane 1) but appears in the supernatant corresponding to the “-Ca2+” sample (lane 2). Consequently, p32 is precipitated with membranes in the presence of Ca2+ and remains in solution in the absence of this cation. It should be noted that without membranes p32 is present independent of the addition of CaCl2.
The membrane pellets were resuspended in EGTA-containing buffer, centrifuged, and the supernatants were analyzed for the presence of p32. As a result, p32 was detected only in the supernatants corresponding to “+Ca2+” sample (lane 3) but not in “-Ca2+” sample (lane 4). This fact indicates that Ca2+-dependent binding of p32 to photoreceptor membranes is reversible.Fig. 2. Ca2+-dependent interactions of p32 with photoreceptor membranes. Before detection p32 was incubated in suspension of photoreceptor membranes in the presence and in the absence of calcium ions (“+Ca2+” and “-Ca2+”, respectively). The suspension was centrifuged and the supernatants were electrophoresed in SDS-containing polyacrylamide gel (lanes 1 and 2, “+Ca2+” and “-Ca2+” samples). The membrane pellet was resuspended in EGTA-containing buffer and centrifuged. The supernatants were electrophoresed in SDS-containing polyacrylamide gel (lanes 3 and 4, “+Ca2+” and “-Ca2+” samples). Positions of molecular mass (kD) markers are shown by arrows; “p32” lane corresponds to the initial p32 preparation.
As a whole, experiments presented in Fig. 2 indicate that the ability of p32 to interact with photoreceptor membranes in a Ca2+-dependent manner is retained after its purification to homogeneity.
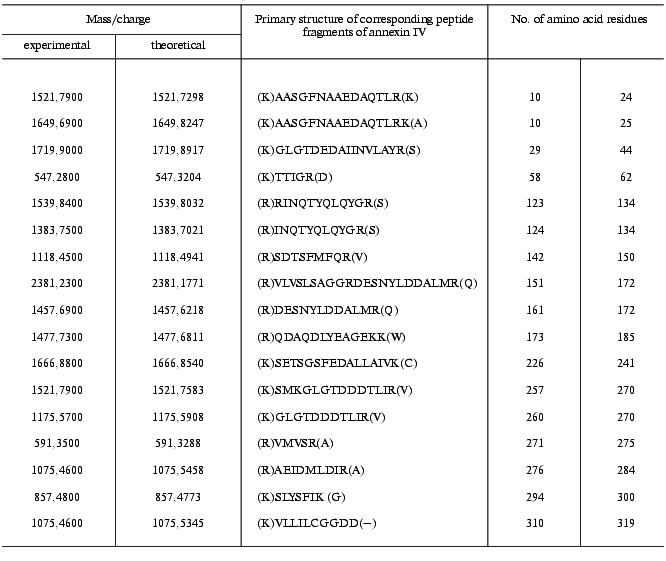
To identify p32, we used MALDI-TOF mass-spectrometry. Preliminarily purified p32 preparation was subject to SDS-PAGE, the protein band was cut from the gel, p32 was eluted, the protein preparation was hydrolyzed by trypsin, and p32 fragments were analyzed by mass-spectrometry. We identified 17 peptide fragments of p32 (table); their comparisons with peptide sets for the known proteins from the amino acid sequence database showed homology with annexin IV, or endonexin (Fig. 3).
Results of mass-spectrometry of peptide fragments obtained by hydrolysis
of p32 by trypsin (experimental conditions are described in
“Materials and Methods”)
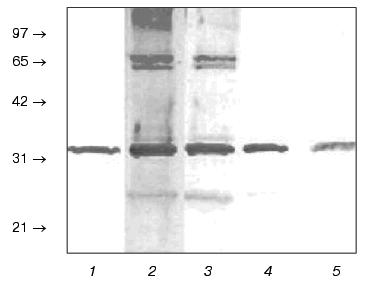
It was shown that annexins earlier isolated from tissues other than retina do not exhibit tissue specificity [15]. We obtained polyclonal (monospecific) antibodies against annexin IV purified from bovine liver and showed that these antibodies cross-reacted with purified p32 (Fig. 4, lane 1) as well as revealed this protein in the initial ROS extract (lanes 2-5). It should be noted that at antibody dilutions less than 1 : 25,000, other protein bands are revealed on immunoblots along with annexin IV. However, these bands are hardly detectable at dilution 1 : 25,000 and completely disappeared at dilution 1 : 50,000. This fact also demonstrates specificity of the observed reaction between annexin IV from retina and antibodies against annexin IV from bovine liver. The ability of the latter to cross-react with p32 from ROS showed in this experiment is also an additional argument that p32 is really annexin IV.Fig. 3. Primary structure of annexin IV from bovine liver. The fragments of amino acid sequence of annexin IV which correspond to p32 peptides according to the data of mass-spectrometry given in the table are underlined.
Thus, for the first time we detected and isolated annexin IV from bovine ROS. In the future we are planning a more detailed study of intracellular localization of annexin IV and to find an intracellular target(s) for this protein; this may elucidate its function in the photoreceptor cell.Fig. 4. Immunochemical detection of annexin IV in ROS extract with polyclonal (monospecific) antibodies against annexin IV from bovine liver. Blots of the purified p32 (1) and bovine ROS extracts at antibody dilutions 1 : 500 (2), 1 : 5000 (3), 1 : 25,000 (4), and 1 : 50,000 (5). Positions of molecular mass markers (kD) are shown by arrows.
It should be added in conclusion that physiological function (or functions) of annexin IV in cells of other types remain unclear. Numerous data on the properties of this protein were obtained exclusively during in vitro experiments (for example, [7]). Detection of annexin IV in photoreceptor cells, a methodologically very convenient object, may help to elucidate its function in vivo.
This study was financially supported by the Russian Foundation for Basic Research (grants No. 00-04-48332 and 02-04-06888) and Ludwig Institute for Cancer Research.
REFERENCES
1.Senin, I. I., Koch, K.-W., Akhtar, M., and
Philippov, P. P. (2002) in Photoreceptors and Calcium (Baehr,
W., and Palczewski, K., eds.) Chap. 5, Landes Bioscience, Georgtown,
USA.
2.Dizhoor, A. M., Chen, C., Olshevskaya, E.,
Sinelnikova, V. V., Philippov, P., and Hurley, J. B. (1993)
Science, 259, 829-832.
3.Nagao, S., Yamazaki, A., and Bitensky, M. W. (1987)
Biochemistry, 26, 1659-1665.
4.Palczewski, K., Subbaraya, I., Gorczyca, W. A.,
Helekar, B. S., Ruiz, C. C., Ohguro, H., Huang, J., Zhao, X., Crabb, J.
B., and Johnson, R. S. (1994) Neuron, 13, 395-404.
5.Kawamura, S., Hisatomi, O., Kayada, S., Tokunaga,
F., and Kuo, C. H. (1993) J. Biol. Chem., 268,
14579-14582.
6.Gorodovikova, E. N., and Philippov, P. P. (1993)
FEBS Lett., 335, 277-279.
7.Raynal, P., and Pollard, H. B. (1994) Biochim.
Biophys. Acta, 1197, 63-93.
8.Schnetkamp, P. P. M., Klompmarkers, A. A., and
Daemen, F. J. M. (1979) Biochim. Biophys. Acta, 552,
379-389.
9.Bradford, M. (1976) Analyt. Biochem.,
72, 248-254.
10.Laemmli, U. K. (1979) Nature, 227,
680-685.
11.Morrisey, J. H. (1981) Analyt. Biochem.,
117, 307-310.
12.Creutz, C. E., Zaks, W. J., Hamman, H. C., Crane,
S., Martin, W. H., Gould, K. L., Oddie, K. M., and Parsons, S. J.
(1987) J. Biol. Chem., 262, 1860-1868.
13.Tikhomirova, N. K. (1990) Biochem.
Int., 22, 31-36.
14.Towbin, H., Staehelin, T., and Gordon, J. (1979)
Proc. Natl. Acad. Sci. USA, 76, 4350-4354.
15.Kaetzel, M. A., Chan, H. C., Dubinsky, W. P.,
Dedman, J. R., and Nelson, D. J. (1994) J. Biol. Chem.,
269, 5297-5302.