REVIEW: Design of Pyridoxal 5´-Phosphate Dependent Catalytic Antibodies
R. M. Khomutov
Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, ul. Vavilova 32, Moscow, 117984 Russia; fax: (095) 135-1405; E-mail: khomutov@genome.eimb.relarn.ru
Received May 7, 2002
Modern approaches for developing antibodies with coenzyme-dependent activities are discussed for pyridoxal 5´-phosphate dependent transformation of amino acid as an example. A new type of antigens analogous to enzyme-substrate compounds is suggested for the production of such antibodies. Approaches for the development of pyridoxal antiidiotypic antibody using analogs of coenzyme-substrate compounds and corresponding apoenzyme complexes are reviewed.
KEY WORDS: transition state analogs, B6 enzymes, haptens, enzyme transition state analogs, coenzyme dependent catalytic antibody
In recent years progress in the implementation of catalytically active antibodies (abzymes) has been to a great extent motivated by studies of enzyme inhibition by compounds initially designed as analogs of intermediate products and transition states of the corresponding enzymatic reactions. The efficiency of these inhibitors proved the ability of modeling the transition states by stable compounds, and therefore allowed applying them as antigens capable of inducing the formation of antibodies with given catalytic properties.
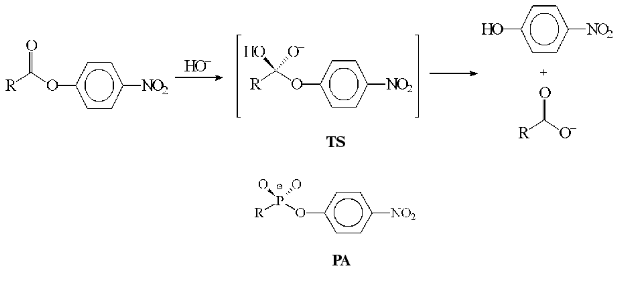
One of the first objects of investigation was the nucleophilic substitution reaction of an unsaturated carbon atom, a well-known process that is based on the addition of a nucleophile to a carbonyl group with a formation of a tetrahedral product, structurally similar to the reaction intermediate state (Fig. 1). As effective inhibitors of the enzymes catalyzing these reactions (amidases, esterases, transacylases), phosphonic acids derivatives being stable analogs of the reactive intermediate compounds were proposed [1, 2]. Antibodies obtained toward this kind of antigens possessed predetermined catalytic properties, which proved the ability of the immune system to generate functionally active antigen replica [3, 4]. Later on the analogs of transition states of other chemical transformations were synthesized and employed for obtaining antibodies able to catalyze more than one hundred different reactions [5, 6]. Nevertheless, only few studies were dedicated to the design of abzymes able to catalyze the reactions performed by coenzyme-dependent enzymes [7].
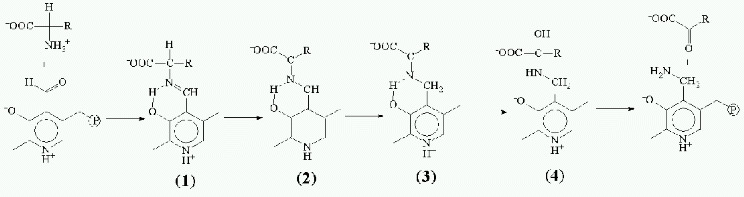
Problems in the design of these abzymes are discussed in this work in terms of reactions performed by enzymes using pyridoxal-5´-phosphate (PLP) as a cofactor and amino acids as substrates. Pyridoxal enzymes (PLP-enzymes) play the key role in amino acid metabolism, catalyzing the transamination, decarboxylation, racemization, and transformations of amino acid radical (aldole cleavage, elimination, and substitution). During the amino acid transformation the formation of covalent coenzyme-substrate compounds with single (4) or double bonds (1, 2, 3) occurs; among those aldimine (1) is a common intermediate compound for all PLP-dependent transformations (Fig. 2). Accordingly, the stable analogs of these compounds may be regarded as haptens, also considering coenzyme ability to perform nonenzymatic amino acid transformations and its active role in catalysis.Fig. 1. Anionic tetrahedral transition state (TS) for the reaction of carboxylic acid ester hydrolysis and phosphonate analog of the TS (PA).
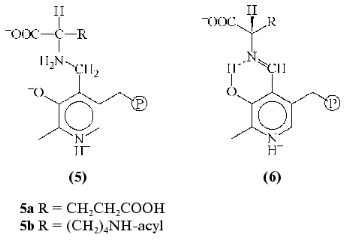
There is only one known type of these analogs, Nalpha-(pyridoxyl-5´-phosphate)-amino acids (PP-amino acids) (5), which are reduced aldimines with a single bond between coenzyme and amino acid fragments (Fig. 3). The substances obtained and studied earlier as efficient inhibitors [8, 9] form stable noncovalent complexes with PLP apoenzymes where analog molecule makes contacts with an active site groups involved in binding of substrate and coenzyme, and also in catalysis [10].Fig. 2. Schematic of amino acid transformation catalyzed by pyridoxal enzymes. Cleavage of aldimine 1 Calpha-H bond causes transamination (shown), racemization, or elimination; break of Calpha-COO- bond leads to decarboxylation, and dissociation of Calpha-R leads to aldole cleavage.
In recent years attempts to design PLP dependent abzymes, a comprehensive study on the preparation and investigation of catalytic properties of 15A9 antibody toward Nepsilon-acyl-Nalpha-(pyridoxyl-5´-phosphate)-L-lysine (5b) as hapten is of greatest interest [11]. The antibody catalyzed a reaction with PLP as a coenzyme--the alpha,beta-elimination of D-beta-chloro-alanine; at the same time, PLP acted as substrate in the transamination reaction, being transformed into pyridoxamine-5´-phosphate as a result of interaction with D-alanine. Catalytic properties of the antibody were significantly different from that of typical pyridoxal enzymes. The PLP was not covalently bound to lysine epsilon-amino group of the antibody as it was in the holoenzyme; there was no acceleration of aldimine 1 formation rate (a necessary step in enzymatic reaction), and the formation of quinonoid tautomer 2, which is the intermediate of all transformations involving cleavage of Calpha-H binding in amino acid, was not detected.Fig. 3. Structures of Nalpha-(pyridoxyl-5´-phosphate)-amino acid (5) and aldimine 1 in the optimal conformation for Calpha-H (6) bond cleavage.
Principally important within the context of the discussed problem was the absence of a specified antibody property such as specificity toward reaction type; however, there was a predicted correlation for known abzymes both in catalyst properties and in hapten structure according to the main idea of abzyme catalysis. In that case, hapten 5a (as a reduced aldimine) was not an analog of the transition state of a certain PLP-dependent reaction, and therefore could not determine the ability of 15A9 antibody to catalyze only alpha,beta-elimination of D-beta-chloro-alanine and transamination of PLP and D-alanine. Hence, the question emerged whether the immune system can generate antibodies able to catalyze other PLP-dependent reactions besides those inherent in 15A9 antibody, and also what should be the hapten structure in order to facilitate the desired PLP-dependent amino acid transformation by the antibody.
In the case of PLP enzymes, the coenzyme-substrate compound is located in the active site in a strictly determined conformation, which governs the reaction specificity and coenzyme participation in catalysis [12]. Though for transamination, racemization, and elimination reactions the optimal position of the cleaved amino acid Calpha-H bond is orthogonal to the plane pyridine cycle of coenzyme (Fig. 3, 6), for the decarboxylation reaction this position is occupied by Calpha-COOH bond. The PP amino acid is being fixed in the active site similarly to the coenzyme-substrate compound, including the orientation of the cleaved bond at the Calpha-atom [10], and this is the only conformation when PP amino acid can be considered as a transition state analog of a particular reaction. In the absence of apoenzyme the amino acid is a number of conformers resulting from free rotation of the molecule fragments around single bonds, which leads to the loss of appropriate Calpha-atom bond orientation and hapten similarity to the transition state of the reaction.
There is an additional uncertainty arising from the antigen due to nonspecific binding of the carrier protein and PP amino acid linked through a flexible bond. The result is the random orientation of hapten conformers relative to the protein and the formation of antigen conformers with different degree of hapten shielding, which are factors influencing the catalytic properties, e.g., of antibody 15A9.
Hence, it is necessary to have the haptens with structure and conformation corresponding to the transition state of a certain PLP-dependent reaction available in order to design PLP abzymes. Currently, there are no such known compounds among the vitamin B6 group; the attempts to design low molecular weight transamination catalysts illustrate [13] that the construction of these analogs is a separate task itself, also considering that PLP modification is often only accompanied by the loss of coenzymatic properties.
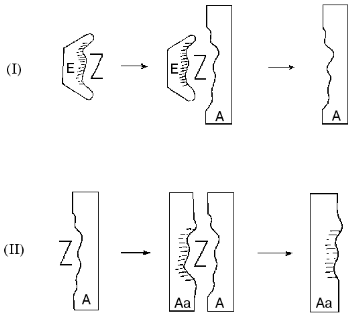
The new approach suggested for the construction of catalytic coenzyme-dependent antibodies is based on the use of stable analogs of enzyme-substrate compounds as antigens, which may be regarded as analogs of enzymatic transition states. In the case of PLP enzymes, these antigens may represent the stable complexes of apoenzymes with analogs of coenzyme-substrate compounds, where the apoenzyme not only induces and sustains the proper analog conformation, but also is functioning as a carrier protein. Catalytically competent state of the active site and certain orientation of analog relative to apoenzyme are implemented in this complex, i.e., the intermediate state of the enzymatic reaction is fixed (Fig. 4, structure E-Z).
For instance, noncovalent adduct of apo-aspartate-aminotransfrease and Nalpha-(pyridoxyl-5´-phosphate)-L-glutamic acid examined earlier may be used as such antigen (Fig. 3, 5a). This compound is structurally close to the enzyme-substrate compound [10] and sufficiently stable for the immunization as it breaks down only under the conditions of protein denaturation [8]. Since the antibody carries the molecular imprint of analog of coenzyme-substrate compound in a required conformation (Fig. 4, structure A), it could also catalyze the formation of PLP aldimine with amino acid and promote the dissociation of Calpha-H bond in aldimine by proper orientation of this bond relative to the pyridine cycle of coenzyme. However, the catalytic activity of this abzyme will probably depend on the proximity and orientation effects, because the transaminase active site groups will be represented by negative replica in structure A.Fig. 4. Formation of catalytically active coenzyme-dependent antibodies. I, II) Immunization steps; E) apoenzyme; Z) analog of coenzyme-dependent compound; A) antibody; Aa) antiidiotypic antibody.
Another, not yet reviewed in literature, possibility of designing coenzyme dependent abzymes can be associated with the representation of the immune system as a network of idiotype-antiidiotype interactions [14]. According to this approach, the antibody obtained toward the active site of an enzyme acts as an antigen during the second stage, which results in a formation of antiidiotypic antibody with a catalytic activity of the original enzyme. This model was successfully implemented (e.g., for acetylcholine esterase [15]), when it was presented that during the immunization there is reproduction of main characteristics and catalytic groups even of a sterically hindered active site located at the bottom of a narrow pocket of enzyme molecule and therefore inaccessible for large low molecular weight modifiers [16]. It is appropriate to note here a possible link between these processes and the appearance of abzymes under autoimmune pathologies [17, 18].
Regarding to PLP holoenzymes, the simple reproduction of this technique seems to be problematic since the risk of losing the coenzyme-binding region during the immunization, and also the natural limitations for coenzyme molecule replication. In the case of PLP apoenzymes, the absence of coenzyme forming an active site may negatively affect on the reflection of complex topology and dynamics of the active site.
However, the antiidiotypical approach appears to be rather promising when the analog of coenzyme-substrate compound is used as a hapten. If the initial antigen is an apoenzyme complex with an analog (Fig. 4, structure E-Z), then the antigen for the subsequent immunization is a complex of analog with an antibody produced after the first immunization (Fig. 4, structure A-Z), which will lead to antiidiotypic antibody (Fig. 4, structure Aa) with mimic of the main characteristics and catalytic groups of the original apoenzyme active site. It can be anticipated that in the case of apo-transaminase the abzyme with PLP as a coenzyme will catalyze the Halpha-atom exchange and the transamination reaction in a similar way as aspartate-aminotransferase.
The interesting possibility is the application of holoenzymes themselves as antigens using this technique (Fig. 4, structure Z, coenzyme). The obtained antibody in a complex with coenzyme will act as antigen for the subsequent immunization with a coenzyme-dependent antiidiotypic antibody as a result. Perhaps similar processes take place under natural conditions, which implies the possibility of emergence of coenzyme-dependent abzymes in autoimmune pathologies.
This approach can be applied similarly, for instance, for acetylcholine esterase. This means that not the enzyme itself acts as an antigen for the first immunization step, but its complex with an inhibitor, the analog of the transition state of the same reaction. The antigen for the second immunization step is the complex of the formed antibody with the same inhibitor (Fig. 4, structure Z, inhibitor). This scheme anticipates that the inhibitor becomes an important additional property of an active site, stabilizing the catalytically competent state of the latter.
The work was financially supported by the Russian Foundation for Basic Research (grants No. 00-15-978844 and 00-04-48242).
REFERENCES
1.Biryukov, A. I., Ishmuratov, B. K., and Khomutov,
R. M. (1978) FEBS Lett., 91, 249-252.
2.Bartlett, P. A., Hunt, J. T., Adams, J. L., and
Gehert, J. C. E. (1978) Bioorg. Chem., 7, 421-430.
3.Tramontano, A., Janda, K. J., and Lerner, R. A.
(1986) Science, 234, 1566-1570.
4.Pollack, S. J., Jacobs, J. W., and Schultz, P. J.
(1986) Science, 234, 1570-1573.
5.Schultz, P. J., and Lerner, R. A. (1995)
Science, 269, 1835-1842.
6.Schultz, P. J., and Lerner, R. A. (1993) Acc.
Chem. Res., 26, 391-395.
7.Hilvert, D. (2000) Annu. Rev. Biochem.,
69, 751-793.
8.Khomutov, R. M., Dixon, H. B. F., Vdovina, L. V.,
Kirpichnikov, M. P., Morozov, Y. V., Severin, E. S., and Khurs, E. N.
(1971) Biochem. J., 124, 99-106.
9.Khurs, E. N., Severin, E. S., Dixon, H. B. F., and
Khomutov, R. M. (1976) Mol. Biol. (Moscow), 10,
897-906.
10.Kirsch, J. F., Eichele, G., Ford, G. C., Vincent,
M. G., Jansonius, J. N., Gehring, H., and Christen, P. (1984) J.
Mol. Biol., 174, 497-525.
11.Gramatikova, S. I., and Christen, P. (1996) J.
Biol. Chem., 271, 30583-30586.
12.Metzler, D. E. (1977) Biochemistry,
Academic Press, Inc., New York-London.
13.Wu, Y., and Ahlberg, P. (1992) Acta Chem.
Scand., 46, 60-72.
14.Jerne, N. K. (1974) Ann. Immun.,
125C, 373-378.
15.Izadyar, L., Friboullet, A., Remy, M. H., Roseto,
A., and Thomas, D. (1993) Proc. Natl. Acad. Sci. USA, 90,
8876-8880.
16.Kolesnikov, A. V., Kozyr, A. V., Alexandrova, E.
S., Koralewski, F., Avalle, B., Demin, A. V., Titov, M. I., Tramontano,
A., Paul, S., Thomas, D., and Gabibov, A. G. (2000) Proc. Natl.
Acad. Sci. USA, 9, 13525-13531.
17.Bronshtein, I. B., Shuster, A. M., Gololobov, G.
V., Gromova, I. I., Kvashuk, O. A., Belostotskaya, K. M., Alekberova,
Z. S., Prokaeva, T. V., and Gabibov, A. G. (1992) FEBS
Lett., 314, 259-263.
18.Shuster, A. M., Gololobov, G. V., Kvashuk, O. A.,
Bogomolova, A. E., Smirnov, I. V., and Gabibov, A. G. (1992)
Science, 256, 665-667.