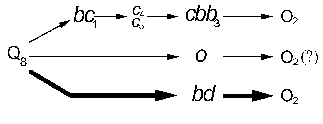Operation of the cbb3-Type Terminal Oxidase in Azotobacter vinelandii
Yu. V. Bertsova and A. V. Bogachev*
Department of Bioenergetics, Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119992 Russia; fax: (095) 939-0338; E-mail: sodiumc@genebee.msu.su* To whom correspondence should be addressed.
Received December 21, 2001; Revision received January 17, 2002
A part of the gene encoding cbb3-type cytochrome oxidase CcoN subunit was cloned from Azotobacter vinelandii and a mutant strain of this bacterium with disrupted ccoN gene was constructed. In contrast to the wild type strain, this one is unable to oxidize cytochromes c4 and c5. Thus, the A. vinelandii respiratory chain is shown to contain cbb3-type cytochrome c oxidase. It is also shown that the activity of this enzyme is not necessary for diazotrophic growth of A. vinelandii at high oxygen concentrations.
KEY WORDS: Azotobacter vinelandii, cytochrome oxidase, cbb3-type cytochrome, respiratory protection
Abbreviations: PCR) polymerase chain reaction; SBP) sub-bacterial particles; TMPD) N,N,N´,N´-tetramethyl-p-phenylenediamine; ApR) ampicillin resistance; ApS) sensitivity to ampicillin; H+/e-) number of protons transferred through the membrane by the respiratory chain enzyme normalized to the number of electrons; KmR) kanamycin resistance; q/e-) charge number transferred through the membrane by the respiratory chain enzyme normalized to the number of electrons; RfR) rifampicin resistance; TcR) tetracycline resistance.
A. vinelandii is a free-living, obligatory aerobic microorganism
capable of nitrogen fixation. Most nitrogen-fixing bacteria are unable
to reduce N2 at high oxygen concentrations because the
nitrogenase complex catalyzing this reaction is irreversibly
inactivated by O2. However, A. vinelandii fixes
molecular nitrogen over a wide range of oxygen concentrations in spite
of the fact that isolated nitrogenase complex of this bacterium is as
sensitive to O2 as that from other microorganisms [1]. To explain such oxygen-resistant nitrogen fixation
of Azotobacter, Dalton and Postgate [2]
advanced the hypothesis that the so-called respiratory protection
mechanism functions in this bacterium. The term “respiratory
protection” means a significant decrease in oxygen concentration
in Azotobacter cytoplasm due to the active work of the
respiratory chain to the level when nitrogenase inactivation does not
occur.
The A. vinelandii respiratory chain includes at least two terminal oxidases, the bd-type quinol oxidase, and o-type cytochrome oxidase [3]. It was shown that mutations in genes encoding cytochrome bd result in the loss of the unique ability of A. vinelandii for molecular nitrogen fixation at high O2 concentrations [4]. Therefore, it was concluded that the respiratory protection of the nitrogenase complex is performed by the bd-type terminal oxidase activity [3, 4]. The operon encoding this enzyme was cloned and sequenced [4], and the enzyme itself was isolated and characterized [5, 6]. In particular, it was shown that activity of this terminal oxidase is coupled with proton potential generation, and the efficiency of this process is 1 H+/e- [7].
In contrast to the bd-type quinol oxidase, the cytochrome oxidase branch of the A. vinelandii respiratory chain is so far poorly studied. It was demonstrated that the process of ubiquinol oxidation by this branch is sensitive to low concentrations of myxothiazol and antimycin [7]. Therefore, it was concluded that this process is realized via bc1-complex. The presence of the following cytochromes c--c4, c5, c551, and c555--was demonstrated in A. vinelandii [8]. The last two types of cytochrome c are minor and their functions are unknown. As for cytochromes c4 and c5, their possible role is to maintain electron transfer from the bc1-complex to cytochrome oxidase. A high TMPD-oxidase activity is typical of A. vinelandii mutant strains lacking genes encoding either cytochrome c4 or cytochrome c5, whereas the mutant strain lacking both these cytochromes c oxidizes TMPD very slowly [9, 10]. These data suggest that there are two alternative electron flows towards the cytochrome oxidase--via cytochrome c4 as well as c5 [9].
Earlier the o-type terminal oxidase was isolated from A. vinelandii [11]. However, the N-terminal sequences in subunits were not determined in the course of this study, and the composition of cofactors of this enzyme also remained unknown. Thus, it remained unknown to what class of terminal oxidases does the o-type oxidase from A. vinelandii belongs. Later Leung and coauthors [12] cloned a part of the A. vinelandii gene (cyoB) probably encoding the o-type terminal oxidase. It was shown that this cloned fragment is homologous to the site of cyoB gene from Escherichia coli encoding the bo-type quinol oxidase subunit. Inactivation of this gene in A. vinelandii resulted in disappearance of the cell spectral characteristics ascribed to the o-type terminal oxidase. In this mutant strain CO binds only to cytochrome bd [12]. These data can also indicate that only two terminal oxidases are present in A. vinelandii. However, dependence of the respiration rate of A. vinelandii cells on oxygen concentration suggests that three terminal oxidases can function in this bacterium [3]. This suggestion is proved by the fact that along with genes encoding bd- and o-type terminal oxidases, A. vinelandii genome seems to contain also a gene homologous to ccoN gene of cbb3-type cytochrome oxidase subunit [13]. The data can indicate that cbb3-type enzyme exists in A. vinelandii as the third terminal oxidase, but this suggestion still needs experimental corroboration. Thus, the main goal of the present work was to study possible functioning of the cbb3-type terminal oxidase in the A. vinelandii respiratory chain and to clarify the role of this enzyme in the respiratory protection mechanism.
MATERIALS AND METHODS
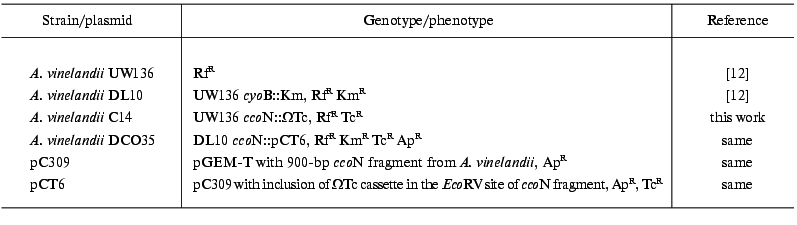
Bacterial strains and growth conditions. A. vinelandii strains used in this work are listed in Table 1. A. vinelandii cells were grown on a thermostatted rotary shaker (250 rpm) at 30°C on modified Burck medium [7].
Table 1. Bacterial strains and plasmids used
in this work
Sub-bacterial particles (SBP) were isolated from A. vinelandii cells as described earlier [14].
Cytochromes c4 and c5 were isolated from A. vinelandii as described in [15].
NADH oxidation by A. vinelandii SBP was measured using a Hitachi 557 spectrophotometer at 340 nm and 30°C. The measurement medium contained 20 mM Hepes (pH 7.5), 60 mM KCl, 2 mM MgSO4, 0.9 µM gramicidin D, and SBP (1-5 µg protein/ml). To calculate the NADH oxidation rate, millimolar extinction coefficient epsilon340 = 6.22 mM-1*cm-1 was used.
Oxidation of cytochromes c by A. vinelandii SBP was measured (at 30°C) using a Hitachi 557 spectrophotometer at 550-535 nm (epsilon550-535 = 17.3 mM-1*cm-1) or at 420-440 nm (epsilon420-400 = 120 mM-1*cm-1). The measurement medium contained 20 mM Hepes (pH 7.5), 60 mM KCl, 2 mM MgSO4, reduced cytochrome c (final concentration of heme C was 5 µM) and SBP (10-50 µg protein/ml). Reduced cytochrome c was obtained by its incubation with ascorbate (5 mM) and subsequent purification from the reductant on a column with Sephadex G-25.
Spectral characteristics of SBP from various A. vinelandii strains were studied using an Aminco DW-2000 spectrophotometer. The measurement medium contained 100 mM KH2PO4, pH 7.0, and SBP (4-6 mg protein/ml). For complete oxidation of cytochromes, SBP were incubated in the presence of 1 mM potassium ferricyanide; to reduce cytochromes, a few crystals of sodium dithionite were added to the sample.
Protein concentration was measured by bicinchoninic acid method [16] using bovine serum albumin as the standard.
Construction of A. vinelandii strain with disrupted synthesis of CcoN protein. An A. vinelandii ccoN fragment was amplified by the polymerase chain reaction (PCR) method using RC3 5´-CGTCTGCGTCCGCTG and H6 5´-CCGGAGTGGACGACGCCGATGGTCAG primers as described in [13]. An amplified fragment of ccoN gene was cloned into pGEM-T vector from Promega (USA) to obtain pC309 plasmid. Then a tetracycline resistance cassette from pHP45 OmegaTc plasmid was incorporated into the unique EcoRV restriction site (positioned approximately 250 bp away from the 5´-end of PCR product). OmegaTc-containing plasmid (pCT6) with the same direction of ccoN gene transcription and tetracycline resistance cassette was sampled. As a result of transformation of A. vinelandii cells by pCT6 plasmid, there was selected a C14 clone (ccoN::OmegaTc) with ApSTcR phenotype typical of an insertion mutation obtained by a double homologous crossing-over event. Localization of mutation in A. vinelandii chromosome was proved by PCR method using RC3 primers (see above) and NC (5´-GAAGGCCAGGGAGACCAC). Such PCR analysis of DNA from C14 clone revealed a unique 2.9-kb product (composed of 2.1- and 0.8-kb components corresponding to the tetracycline resistance cassette and PCR fragment of ccoN gene). We failed to detect the 0.8-kb PCR product typical of the wild type strain.
Mutagenesis of DL10 A. vinelandii cells (cyoB::Km) was performed analogously; a DCO35 strain (cyoB::Km ccoN::pCT6) in which ccoN inactivation occurred via the integration of pCT6 plasmid due to a single crossing-over, was thus obtained.
Competent A. vinelandii cells were obtained and transformed as described in [17].
Nucleotide sequencing was performed in the Human Genome Sequencing and Mapping Group of the Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, using an Applied Biosystems ABI 373A automatic sequencer.
The DNA sequence determined in this study was deposited into GenBank (No. AF456880).
RESULTS AND DISCUSSION
Study of the cbb3-type cytochrome oxidase from Rhodobacter capsulatus demonstrated that gene fragment of this enzyme is also present in genomes of many other bacteria [13]. Among them, it was shown that A. vinelandii chromosome contains the ccoN fragment (encoding cbb3-type cytochrome oxidase CcoN subunit in R. capsulatus) [13]. The data suggested that such terminal oxidase possibly functions also in A. vinelandii. To test this hypothesis, a mutant strain of A. vinelandii with disrupted synthesis of CcoN protein was constructed. For this, the ccoN gene fragment was amplified by PCR method as described in [13], and the products were separated by gel electrophoresis. The 900-bp band was isolated, cloned into pGEM-T vector (thus obtaining pC309 plasmid), and partially sequenced. Analysis of the resulting sequence using a BLASTX program showed that amino acid sequence corresponding to a certain nucleotide sequence is homologous to cbb3-type terminal oxidase CcoN subunit from various bacteria. Maximal homology (99%) was found in CcoN protein analog from Pseudomonas aeruginosa. Thus, it was the ccoN gene fragment that we cloned from A. vinelandii. A partial nucleotide sequence of this gene was deposited into GenBank (No. AF456880).
The tetracycline resistance cassette was incorporated into the cloned fragment, and this resulted in pCT6 plasmid construction. To obtain the A. vinelandii strain with inactivated ccoN gene, cells of this bacterium were transformed by pCT6 plasmid (as all plasmids containing ColE1-replicon, this plasmid is unstable in A. vinelandii cells). A C14 clone with ApSTcR phenotype typical of mutation obtained due to a double crossing-over was sampled. Localization of the insertion mutation in A. vinelandii ccoN gene was proved by PCR analysis (see “Materials and Methods”). Thus, we constructed the A. vinelandii strain in which ccoN gene contains tetracycline resistance cassette; this should result in disruption of CcoN protein synthesis.
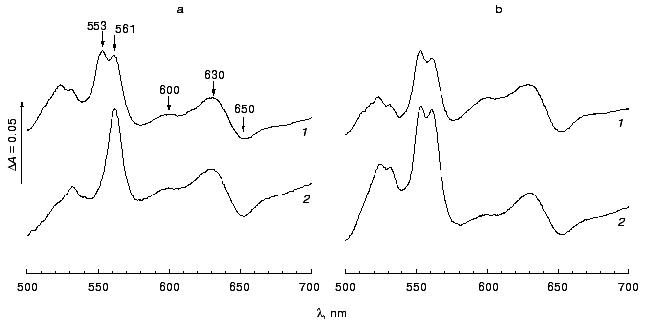
If A. vinelandii CcoN protein is a subunit of the functioning cbb3-type terminal oxidase, then ccoN gene mutation should result in significant decrease in the cytochrome c oxidase activity in this microorganism, since all the so far described cbb3-type oxidases are cytochrome oxidases. As shown in Fig. 1a, the dithionite-reduced minus air-oxidized difference spectrum of SBP isolated from the wild type A. vinelandii strain indicates the presence of cytochromes c, b, and d in membrane preparations of this bacterium. However, a peak at 553 nm is essentially absent from a similar (dithionite-reduced minus air-oxidized) difference spectrum of SBP from the CcoN-mutant A. vinelandii strain (C14); this may indicate a very low content of cytochromes c in these membrane preparations (Fig. 1a). Nonetheless, dithionite-reduced minus ferricyanide-oxidized difference spectra of SBP from these two strains are practically the same (Fig. 1b). The data mean that cytochromes c are present in SBP from the CcoN-mutant A. vinelandii strain, but in isolated SBP they are in reduced form, that is, in the mutant strain oxidation of cytochromes c by air oxygen is hindered, and they can be oxidized only in the presence of ferricyanide. The data indicate that the active cytochrome oxidase is absent from SBP from the A. vinelandii mutant strain.
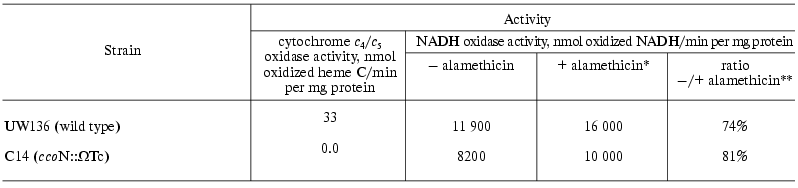
To prove this suggestion, the fraction of cytochromes c4 and c5 was isolated from A. vinelandii cells, and the rate of oxidation of these cytochromes by SBP from various strains of the studied bacterium was measured. In accord with the previous observations [11], it was shown that SBP isolated from the A. vinelandii wild type strain (UW136) cells are able to oxidize cytochromes c4 and c5 (Table 2). The DL10 A. vinelandii strain mutant in the o-type terminal oxidase (cyoB::Km) demonstrated approximately the same cytochrome c4/c5 oxidase activity as the wild type strain (data not presented). The cytochrome c oxidase activity of these strains was completely inhibited in the presence of 20 µM KCN, whereas NADH oxidase activity was only partly sensitive (~15% inhibition) to such cyanide concentration, because NADH oxidase activity of A. vinelandii SBP is performed mainly due to the functioning of the bd-type “cyanide-resistant” quinol oxidase [7]. However, we failed to detect oxidation of cytochromes c4 and c5 by the CcoN-mutant A. vinelandii strain (C14), although the rate of NADH oxidation by SBP of this strain was approximately the same as that of the wild type strain (Table 2). The same results were obtained using cytochrome c from horse heart; the only difference was that the oxidation rate of this cytochrome was significantly lower compared with that of A. vinelandii cytochromes c4 and c5.Fig. 1. Dithionite-reduced minus air-oxidized (a) and dithionite-reduced minus ferricyanide-oxidized (b) difference spectra of sub-bacterial particles isolated from the wild type A. vinelandii strain (1) and C14 mutant strain (2). All spectra are normalized to protein concentration 5 mg/ml.
Table 2. The oxidation rate of cytochromes
c4/c5 and NADH by sub-bacterial
particles from various A. vinelandii strains (results of a
typical experiment are presented)
*NADH oxidase activity in the presence of 17 µg/ml
alamethicin.
**The ratio of NADH oxidase activity in the absence of
alamethicin to this activity in the presence of alamethicin (%).
It is well known that NADH oxidation proceeds on the inner surface of the bacterial cytoplasmic membrane, whereas cytochrome c is oxidized on the outer surface of this membrane [18]. Thus, we had to ascertain that the ratio of “right-side out” and “inside out” particles in membrane preparations from the C14 mutant strain is not changed compared with the wild type strain. As seen from the Table 2, stimulation of NADH oxidase activity by alamethicin was approximately the same in SBP from the mutant strain and in those from the wild type strain. Alamethicin is able to form channels of rather large size in the membrane in order to provide its permeability for NADH [19]. In the absence of alamethicin, only “inside out” particles catalyze NADH oxidation, whereas in the presence of this channel-former both populations of particles are catalysts (this stimulation of NADH oxidase activity by alamethicin cannot be rationalized by the effect of respiratory control, because all measurements were performed in the presence of gramicidin). Similar stimulation of NADH oxidase activity by alamethicin (Table 2) means that orientation of SBP isolated from the A. vinelandii mutant strain does not differ from orientation of SBP from the wild type strain. Thus, the lack of cytochrome c oxidase activity in SBP from the C14 mutant strain can be explained only by the lack of the active cytochrome oxidase in this strain.
The data indicate that the A. vinelandii respiratory chain contains the active cbb3-type cytochrome oxidase. Since ccoN gene mutation results in complete loss of cytochrome c oxidase activity, our data also indicate that all other terminal oxidases of A. vinelandii are quinol oxidases.
It should be noted that the oxidation rate of cytochromes c by SBP from A. vinelandii is significantly lower compared with the oxidation rate of NADH by the same SBP. In spite of the fact that the measured rate of cytochrome c oxidation is significantly underestimated due to a small content of “right-side out” particles in our membrane preparations (approximately fivefold, see Table 2), one can conclude that the cytochrome oxidase branch of the A. vinelandii respiratory chain is a minor component compared with the bd-type quinol oxidase.
As shown earlier, mutations in genes encoding the bd-type quinol oxidase result in disruption of diazotrophic growth of A. vinelandii at high oxygen concentrations [4]. However, the A. vinelandii strains with disrupted synthesis of o-type terminal oxidase are able to fix molecular nitrogen at high oxygen concentrations [12]. In this study we demonstrated that ccoN gene mutation (C14 strain) along with the double ccoN cyoB mutation (DCO35 strain) do not effect ability of A. vinelandii for diazotrophic growth at high oxygen concentrations (data not presented here). Thus, one can conclude that the o- and cbb3-type terminal oxidases (in contrast to the bd-type quinol oxidase [4]) are not necessary for functioning of the respiratory protection mechanism of the nitrogenase complex.
Our results indicate that the A. vinelandii respiratory chain contains cbb3-type cytochrome oxidase along with the o- and bd-type terminal oxidases [4, 12]. Accounting for the earlier results [4, 7, 10, 12], the terminal site of A. vinelandii respiratory chain can be presented as the following scheme (Fig. 2). The bd-type quinol oxidase possesses low energy-conserving efficiency (1 q/e-) [7], a high rate of oxygen reduction and is a necessary component of the respiratory protection [4]. The cbb3- and o-type terminal oxidases are to possess high energy-conserving efficiency (2 q/e-, since they belong to superfamily of heme-copper oxidases [20]) and demonstrate relatively low activity with A. vinelandii. The physiological role of the two latter terminal oxidases seems to be energy supply of A. vinelandii cells during growth at low oxygen concentrations.
The authors are grateful to Professor R. Poole for a kind donation of A. vinelandii strains. This study was financially supported by the Russian Foundation for Basic Research (grant No. 01-04-48147) and special assistance for young scientists (grant No. 02-04-0610-mas).Fig. 2. Scheme of the terminal site of A. vinelandii respiratory chain.
REFERENCES
1.Yates, M. G. (1988) in The Nitrogen and Sulphur
Cycles (Cole, I. A., and Ferguson, S. I., eds.) Cambridge,
Cambridge University Press, Vol. 42, pp. 386-416.
2.Dalton, H., and Postgate, J. R. (1968) J. Gen.
Microbiol., 54, 463-473.
3.Poole, R. K., and Hill, S. (1997) Biosci.
Rep., 17, 303-317.
4.Kelly, M. J. S., Poole, R. K., Yates, M. G., and
Kennedy, C. (1990) J. Bacteriol., 172, 6010-6019.
5.Junemann, S., and Wrigglesworth, J. M. (1995) J.
Biol. Chem., 270, 16213-16220.
6.Kolonay, J. F., Moshiri, F., Gennis, R. B.,
Kaysser, T. M., and Maier, R. J. (1994) J. Bacteriol.,
176, 4177-4181.
7.Bertsova, Y. V., Bogachev, A. V., and Skulachev, V.
P. (1997) FEBS Lett., 414, 369-372.
8.Jones, C. W., and Redfearn, E. R. (1967)
Biochim. Biophys. Acta, 143, 340-353.
9.Ng, T. C. N., Laheri, A. N., and Maier, R. J.
(1995) Biochim. Biophys. Acta, 1230, 119-129.
10.Rey, L., and Maier, R. J. (1997) J.
Bacteriol., 179, 7191-7196.
11.Jurtshuk, P., Mueller, T. J., and Wong, T. Y.
(1981) Biochim. Biophys. Acta, 637, 374-382.
12.Leung, D., van der Oost, J., Kelly, M., Saraste,
M., Hill, S., and Poole, R. K. (1994) FEMS Microbiol. Lett.,
119, 351-358.
13.Thöny-Meyer, L., Beck, C., Preisig, O., and
Hennecke, H. (1994) Mol. Microbiol., 14, 705-716.
14.Bertsova, Y. V., Bogachev, A. V., and Skulachev,
V. P. (1998) Biochim. Biophys. Acta, 1363, 125-133.
15.Pettigrew, G. W., and Brown, K. R. (1988)
Biochem. J., 252, 427-435.
16.Smith, P. K., Krohn, R. I., Hermanson, G. T.,
Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K.,
Goeke, N. M., Olson, B. J., and Klenk, D. C. (1985) Analyt.
Biochem., 150, 76-85.
17.Bertsova, Y. V., Bogachev, A. V., and Skulachev,
V. P. (2001) J. Bacteriol., 183, 6869-6874.
18.Skulachev, V. P. (1988) Membrane
Bioenergetics, Springer, Berlin.
19.Grivennikova, V. G., Kapustin, A. N., and
Vinogradov, A. D. (2001) J. Biol. Chem., 276,
9038-9044.
20.Wikström, M., Bogachev, A., Finel, M.,
Morgan, J. E., Puustinen, A., Raitio, M., Verkhovskaya, M., and
Verkhovsky, M. I. (1994) Biochim. Biophys. Acta, 1187,
106-111.