From Biochemistry of Amino Acid Metabolism to Molecular Enzymology. In Recognition of the 100th Anniversary of the Birth of A. E. Braunstein, Member of the Academy of Sciences of the USSR and the Academy of Medical Sciences of the USSR
E. S. Severin and Yu. N. Khomyakov
Research Center of Molecular Diagnostics and Therapy, Simferopolskii Bulvar 8, Moscow, 113149 Russia; fax: (095) 113-2633; E-mail: e.severin@mtu-net.ruReceived April 26, 2002
May 26, 2002 was the 100th anniversary of the birthday of an outstanding biochemist of our time--Alexander Evseevich Braunstein--who in 1937 discovered a reaction of enzymatic transamination and later laid the groundwork for molecular enzymology as a laboratory head in the Institute of Molecular Biology, Academy of Sciences of the USSR. A. E. Braunstein graduated in 1926 from the Kharkov State Medical Institute and started his scientific activity under the supervision of V. A. Engelhardt in the Biochemical Institute, Narkomzdrav of the USSR, in Moscow. In 1936 he took charge of the Laboratory of Intermediate Nitrogen Metabolism (VIEM) which from 1945 formed a part of the Institute of Biological and Medical Chemistry, the Academy of Medical Sciences of the USSR.
A reversible reaction of enzymatic transfer of the amino group from alpha-amino acids to alpha-keto acids, transamination, which was found by A. E. Braunstein in 1937 together with M. G. Kritsman is fundamental for understanding pathways of nitrogen biological assimilation and dissimilation. During that period in the world of science there was enthusiasm for studies of newly found miraculous molecules, vitamins. The comprehension of their secrets seemed to solve the problem of longevity and health. After the finding in 1931 of vitamin B2 nonhomogeneity and the discovery of vitamin B6 [1], many scientists throughout the world supposed that the latter should be a coenzyme because in 1938 it was shown by Kuhn to be bound to proteins [2].
By this time Braunstein with colleagues had already performed experiments to find a donor of amino acids during the resynthesis of AMP from inosinic acid in muscle. On investigating works of other authors on this problem, Braunstein supposed that the amino group of glutamic and aspartic acids could be used as a donor. Experiments under aerobic and anaerobic conditions designed to check this hypothesis and further attempts to find other acceptors of the glutamate nitrogen were performed. As a result, during the interaction between aminophenylacetic and pyruvic acids the generation of equimolar quantities of benzaldehyde, carbon dioxide, and alpha-alanine was found:
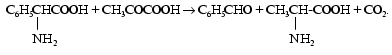
In 1937 in Braunstein's laboratory a reversible translocation of the amino group was found under biological conditions between glutamic and aspartic acids; it could be in general terms described as a transfer of the amino group together with proton and two electrons from an amino acid onto a keto acid [3]. This reaction is now known as transamination and enzymes responsible for its catalysis are called transaminases, or aminotransferases.
From 1945 Braunstein and his coworkers paid attention mainly to interpretation of the molecular bases of enzymatic transformations of amino acids. He found that the vitamin B6 derivative pyridoxal-5-phosphate (PP) was involved as a coenzyme in various enzymatic reactions of amino acids. And the transamination reaction in the general scheme of integration of nitrogen metabolism occupied the central place; the relation of amino acid transformations with the metabolism of carbohydrates and lipids through transamination reaction was doubtless.
The priority of A. E. Braunstein in the discovery of transamination is recognized all over the world, and his studies on nitrogen metabolism integration are widely extended. In 1941 Braunstein was awarded the State Prize for the discovery of the transamination reaction, and the main results of his research during that period were summarized in his book “Biochemistry of Amino Acid Metabolism” published in 1949. In the foreword to his book Braunstein wrote: “Biochemistry owes much valuable research and important findings to Russian scientists, beginning from I. P. Pavlov, M. Nentsky, A. Ya. Danilevsky, I. P. Borodin, D. N. Pryanishnikov, V. S. Gulevich, N. D. Zelinsky, N. I. Gavrilov, N. N. Ivanov, E. S. London, and their students... In the current development of special problems in physiology and biochemistry of amino acid metabolism a significant role belongs to the works and ideas of a number of Soviet scientists: S. S. Salazkin, B. I. Zbarsky, S. Ya. Kaplansky, S. E. Severin, Yu. M. Gefter, A. M. Utevsky, D. L. Ferdman, A. N. Parshin, M. G. Kritsman, A. E. Braunstein, and their coworkers.” Detailed knowledge of the biochemical behavior of amino acids is now a key for solution of many of the most significant problems in comparative an evolutionary physiology, microbiology, pharmacology and chemotherapy, clinical medicine, biochemical technology, etc. The book “Biochemistry of Amino Acid Metabolism” for a long time was a manual for every Soviet biologist and was surprising by its encyclopedic content and the wide range of coverage of experimental data in this field.
The general theory of action of pyridoxal-5-phosphate-dependent enzymes developed by A. E. Braunstein and M. M. Shemyakin (1952-1954) [4] is of great importance. The aldehyde group of PP can produce with amino acids Schiff bases (otherwise called aldimines or azomethins), and in these compounds an electron from the alpha-carbon atom of the amino acid is shifted through the system of coupled double bonds to the electrophilic nitrogen atom in the pyridine base of the coenzyme. In turn, the decrease in the electron density results in polarization and rupture of the bond with subsequent generation of a carbanion and secondary rearrangements. The bond between the alpha-carbon of the amino substrate and the alpha-proton is broken, with a subsequent prototropic rearrangement in the azomethin with production of a ketimine which later is hydrolyzed to pyridoxamine phosphate and alpha-keto acid. Then the back reaction occurs: the pyridoxamine form of transaminase interacts with the new keto acid that results in the generation of a new amino acid and a recovery of the initial enzyme.
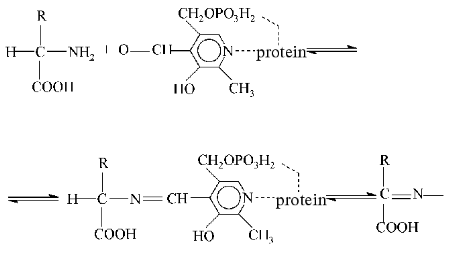
(The character and specificity of these reactions are determined by structures of the protein molecule and of the amino acid as a substrate)
Two years after the Braunstein-Shemyakin theory had been published works appeared in other countries which virtually repeated the results of the Russian authors. E. E. Snell and D. E. Metzler studied the interaction of pyridoxal with amino acids using model experiments in aqueous medium at physiological pH values. The reaction was shown to result in the production of pyridoxamine and keto acid [5]. The authors interpreted the data also based on the influence of the system of coupled double bonds on the electron density at the alpha-carbon of the amino acid.
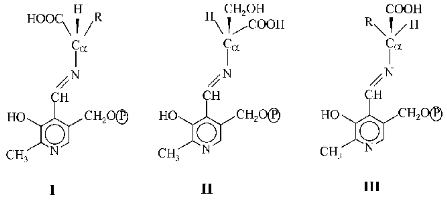
Conditions which determined the direction of these reactions were also studied by Braunstein's students. These conditions were found to be associated with stereochemistry of the protein moiety of the enzyme. The spatial organization of the molecules determined not only the rate but also the direction of reactions with their involvement. Afterwards the assertion of Braunstein and Shemyakin that specific structural features of the protein apoenzyme influenced the selective character of reactions in the active sites of pyridoxal enzymes was developed and confirmed by a number of studies [6, 7] on the specificity of effects of these enzymes. The direction of reactions was found to be determined by conformation of substituents at the alpha-carbon-nitrogen bond in the PP imine generated. The bond to be broken should be located at a right angle to the plane of the pyridine ring. This orientation provides a maximal overlapping of orbitals of the sigma-bond activated and of pi-bonds of the coupled system. Therefore, different conformers shown below were characterized by differently located breaks. The bond Calpha-H should be broken in the conformer I, whereas in the conformers II and III the bonds Calpha-CH2OH and Calpha-COOH, respectively, should be broken.
The main works of A. E. Braunstein and his students in molecular enzymology and action mechanism of pyridoxal enzymes were performed in the Institute of Molecular Biology, the Academy of Sciences of USSR, where he was invited by Academician V. A. Engelhardt in 1959. Engelhardt, Director of the Institute of Physicochemical and Radiation Biology (later called the Institute of Molecular Biology of the Academy of Sciences of the USSR), deemed it necessary that the Institute should include representatives of three main specialties: biochemists, physicists, and chemists. In the book “Cognition of Life Phenomena” the author describes his concept of the new institute as follows: “It was a great satisfaction for me and a happy situation for the institute that a significant number of middle age and relatively young researchers enter our cooperation even in the early stages of the institute's existence. The cooperation with my former students, such as A. A. Baev and A. E. Braunstein, was especially fruitful. They were my young companions-in-arms long years ago and now became a strong base of the Institute”. And as a result of realization of this concept, during the organization of the new institute, in the Laboratory of Chemical Bases of Catalysis headed by Braunstein talented biochemists (E. V. Goryachenkova, Yu. M. Torchinskii, O. L. Polyanovskii, B. S. Sukhareva, etc.) and chemists (R. M. Khomutov, M. Ya. Karpeiskii, E. S. Severin) were united and also younger researchers who worked in the adjacent fields. This cooperation of scientists of different specialties resulted in works supervised by A. E. Braunstein on the isolation, purification, and studies on properties of various pyridoxal enzymes, on topography of the active sites of these enzymes, in establishment of the complete primary structure of aspartate-glutamate transaminase (in cooperation with Academician Yu. A. Ovchinnikov) and crystallization and the subsequent establishment of the three-dimensional structure of aspartate-glutamate transaminase from chicken heart (in cooperation with Academician B. K. Vainstein). An important step in the progress of molecular enzymology was a development of the concept of selective inhibitors of pyridoxal enzymes (R. M. Khomutov, E. S. Severin) and of the fundamentally new dynamic model of functioning of the active site of aspartate-glutamate transaminase and of its coenzyme (M. Ya. Karpeiskii, V. I. Ivanov).
Aspartate transaminase was shown to have two forms, aldiminic and pyridoxaminic, each with a characteristic absorption spectrum. In the aldiminic form the carbonyl group of the PP coenzyme is bound to the epsilon-amino group of lysine as a Schiff base. In the enzyme pyridoxaminic form the coenzyme is present as pyridoxamine-5´-phosphate and displays a high affinity for the apoenzyme. During the enzymatic transamination the amino acid is bound to the aldiminic bond of PP with lysine with subsequent generation of a Schiff base between PP and the amino acid and with release of the free epsilon-amino group of lysine. The prototropic rearrangement of aldimine into ketimine and the subsequent hydrolysis of the latter result in production of the corresponding keto acid and the pyridoxamine form of the enzyme. During the back reaction pyridoxamine phosphate produces a Schiff base with the keto acid, and the reaction products in this case are the amino acid and PP. The enzymatic transamination goes by the “shuttle” mechanism, and the enzyme is by turn in the form of aldehyde or amine [8]. Various approaches have been used for the investigation of different stages of the transamination mechanism, including careful studies on intermediate products of the reaction.
As an intermediate product of transamination, ES-complex was studied [9] which was produced on the initial stage of the reaction as a result of the protonated amino acid binding as a substrate to the non-protonated aldimine of the enzyme. In the next stage the proton was transferred from the amino group of the amino acid to the enzyme aldimine because an undivided electron pair on the amino acid nitrogen was required for the nucleophilic addition. Then the amino acid was involved in the generation of Schiff base, which in the next stage was transformed to a quinoid structure which was a tautomeric form of carbanion. The limiting stage of the whole process was the prototropic rearrangement and ketimine production. The terminating stage was the release of the reaction product keto acid and the production of the pyridoxaminic form of the enzyme.
Studies on stereochemistry of enzymatic catalysis have shown that changes in the PP-enzyme conformation under the influence of some substances, e.g., esters of aminocarbohydroxaminic acid, play a determining role in their inhibitory effect on the catalysis [8]. Great attention was paid to studies on physicochemical features of specific inhibitors of PP-enzymes by the principle of selective inhibition.
It is difficult to overestimate the significance of works performed under the supervision and with the immediate participation of A. E. Braunstein. The theory Braunstein and Shemyakin was the first in the history of science which allowed atom and electron concepts to be used for explanation of PP-associated enzymatic transformations of amino acids during their synthesis and metabolism. This theory has allowed us to consider from the same standpoint mechanisms of amino acid transformations with involvement of transaminases, racemases, alpha- and beta-decarboxylases, and also alpha-, beta-, and gamma-substitutions and elimination in amino acids. In addition to direct theoretical and applied significance, this theory is important also because it provided a qualitatively new level of not only enzymology but of biochemistry in total and determined the development of new methodological approaches.
Even in 1974 in his plenary lecture at the III All-Union Biochemical Congress, Braunstein said: “The development of biochemistry... in the XX century went through some stages. The static descriptive biochemistry gave birth in parallel to two lines: to dynamic biochemistry, i.e., the description of metabolic processes and dissection of them to the simplest enzymatic links, and to functional biochemistry, i.e., the finding relations between metabolic transformations and physiological acts under normal and pathological conditions, in other words, to biological and medical chemistry. The research pathways ascended from small molecules to the most complicated polymers and deepened from chemistry of large morphological structures to ultramicroscopic ones. And the cognition moved, respectively, from the phenomenology of changes induced by enzymes yet undescribed as chemical entities to studies on the structure and functions of individual proteins, enzymes, by precise physical and chemical methods, i.e., to that we call molecular enzymology” [10].
A line in biology and medicine which appeared in the XXI century and uses modern computerized technologies and leans upon recent findings in molecular genetics and cytology is called molecular medicine. Drugs of new generations allow us to influence the human body on the molecular level. The biological and medical science which is now developing at a fantastic rate habitually uses concepts of the atom-molecule theory. Catalytic reactions cannot be studied without computer-aided modeling of space-stoichiometric interrelations between the substance molecules at the consecutive stages of reactions. However, one should remember that the foundations of this line were laid long before the computer era by such scientists as A. E. Braunstein, whose 100th anniversary is marked in 2002 by Russian and world science.
REFERENCES
1.Gyorgy, P. (1935) Biochem. J., 29,
741-767.
2.Kuhn, R., and Wendt, G. (1938) Ber. Chem.
Ges., 71, 780-1534.
3.Braunstein, A. E., and Kritsman, M. G. (1937)
Biokhimiya, 2, 859.
4.Braunstein, A. E., and Shemyakin, M. M. (1953)
Biokhimiya, 18, 393.
5.Metzler, D. E., Ikawa, M., and Snell, E. E. (1954)
J. Am. Chem. Soc., 76, 648.
6.Maley, J. R., and Bruice, T. C. (1968) J. Am.
Chem. Soc., 90, 2843.
7.Dunathan, H. C. (1966) Proc. Natl. Acad. Sci.
USA, 55, 712.
8.Khomutov, R. M., Severin, E. S., Kovaleva, G. K.,
Gulyaev, N. N., Gnuchev, N. V., and Sashenko, L. P. (1968) in
Chemistry and Biology of Pyridoxal Catalysis [in Russian],
Nauka, Moscow.
9.Braunstein, A. E. (1970) FEBS Symp.,
18, 101.
10.Braunstein, A. E. (1974) Proc. III All-Union
Biochem. Congr., Vol. 3-4, Institute of Molecular Biology,
Moscow.