REVIEW: Rhodopsin Receptors of Phototaxis in Green Flagellate Algae
O. A. Sineshchekov* and E. G. Govorunova
School of Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; E-mail: o.sineshchekov@mtu-net.ru* To whom correspondence should be addressed.
Received May 10, 2001; Revision received July 22, 2001
Green flagellate algae are capable of the active adjustment of their swimming path according to the light direction (phototaxis). This direction is detected by a special photoreceptor apparatus consisting of the photoreceptor membrane and eyespot. Receptor photoexcitation in green flagellates triggers a cascade of rapid electrical events in the cell membrane which plays a crucial role in the signal transduction chain of phototaxis and the photophobic response. The photoreceptor current is the earliest so far detectable process in this cascade. Measurement of the photoreceptor current is at present the most suitable approach to investigation of the photoreceptor pigment in green flagellate algae, since a low receptor concentration in the cell makes application of optical and biochemical methods so far impossible. A set of physiological evidences shows that the phototaxis receptor in green flagellate algae is a unique rhodopsin-type protein. It shares common chromophore properties with retinal proteins from archaea. However, the involvement of photoelectric processes in the signal transduction chain relates it to animal visual rhodopsins. The presence of some enzymatic components of the animal visual cascade in isolated eyespot preparations might also point to this relation. A retinal-binding protein has been identified in such preparations, the amino acid sequence of which shows a certain homology to sequences of animal visual rhodopsins. However, potential function of this protein as the phototaxis receptor has been questioned in recent time.
KEY WORDS: phototaxis, photoreceptor, rhodopsin, signal transduction, photoreceptor current, photoelectric processes, green flagellate algae, Chlamydomonas, Haematococcus
Motile microorganisms are capable of responding to light stimuli by changes in their behavior, which leads to their accumulation under optimal conditions. The most advanced type of photoinduced behavioral responses is phototaxis, by which cells actively adjust the swimming path with respect to the direction of light incidence [1]. The ability to detect this direction assumes either the presence of two spatially separated receptors in the cells, or (as in green flagellate algae) comparison of the illumination of the only receptor at different orientations of the cell in the environment--the so-called “two-instant mechanism”. Accumulation of the cells in the area with optimal illumination is provided by regulation of the phototaxis sign, i.e., the movement toward the light source (positive phototaxis), or away from it (negative phototaxis). Besides phototaxis, more primitive behavioral responses are known that are driven by a change in the light intensity regardless of the light direction. Light-dependent alteration of the average swimming speed is referred to as photokinesis, whereas a short stop and/or a sudden change in the direction of movement in response to a step light stimulus--as photophobic response [1].
This purely descriptive classification does not, however, take into account the diversity of signal transduction mechanisms for these responses, which might involve photodestructive processes, light energy conversion (in phototrophs) or specialized photoreception [2, 3]. In green flagellate algae photoregulatory responses of all three types are mediated by a specific photoreceptor system, although processes of photosynthesis take part in regulation of the phototaxis sign [4, 5].
In green flagellates, photoexcitation of the receptor triggers a cascade of rapid electrical phenomena in the cell membrane, which plays a key role in the signal transduction chain for phototaxis and the photophobic response [6-8]. The photoreceptor current is the earliest so far detected event in light regulation of behavior in green flagellate algae. Extremely low concentration of photoreceptor molecules in the presence of abundant photosynthetic pigments significantly complicates their detection by spectroscopic techniques in vivo, as well as its isolation and biochemical purification. This makes electrophysiological methods the most adequate of all currently available approaches not only to investigation of the signal transduction mechanisms for photoregulation of behavior in green flagellates, but also to in vivo studies on the photoreceptor pigments involved.
The body of information obtained by measurement of photoinduced behavioral and electric responses in green flagellate algae leads to the conclusion that their phototaxis receptor is a unique rhodopsin-type protein. The most direct evidence in proof of this notion is restoration of the ability to phototax and generate photoreceptor currents in blind carotenoid-deficient Chlamydomonas mutants after the addition of exogenous retinal [9, 10]. Comparative analysis of the efficiency of various retinal isomers and analogs in experiments with such mutants established the chemical nature of the native chromophore of the phototaxis receptor.
Substantial effort has been invested in identification of genes encoding the opsin part of the photoreceptor pigment in green flagellate algae, since cloning of these genes and their expression in model systems appears to be the most promising (if not the only possible) means to obtain preparative amounts of the photoreceptor proteins. Genes encoding retinal-binding proteins have been found in Chlamydomonas [11] and Volvox [12]. However, at present there are doubts that these proteins have a receptor function [13]. Therefore, search for receptor opsin genes still represents one of the most urgent problems at the present state of research into phototaxis in green flagellate algae.
STRUCTURE AND FUNCTION OF THE PHOTORECEPTOR APPARATUS
A distinct photoreceptor apparatus is employed to track the direction of light in phototactic algae. The structural organization of this apparatus provides the spatial anisotropy of light absorption by photoreceptor molecules. Part of this apparatus that is visible under a light microscope is called the eyespot, or stigma, and plays a role of a shading device. An accessory role of the eyespot is confirmed by the phototactic ability of eyespot-deficient mutants.
In the model microorganism Chlamydomonas reinhardtii, it is ~1 micron in diameter and has a lateral position in the cell [14]. The eyespot position with respect to the plane of flagellar beating is strictly defined by the structural association of the eyespot with the flagellar roots [15]. In Chlorophyceae, the eyespot is part of the chloroplast and consists of one to several layers of carotenoid globules often subtended by thylakoid membranes [15-17].
The photoreceptor pigment is thought to be concentrated in the small area of cell membranes directly adjacent to the eyespot--in the plasma membrane or in the outer chloroplast envelope. Localization of the photoreceptor pigment in the eyespot area is most directly supported by the finding that the photoreceptor current can only be recorded from this part of the cell [18, 19]; moreover, this current is also found in “excised eyes”--eyespot-containing vesicles detached from the cells [20, 21].
Other evidences of such localization are the fixed orientation of the chromophores in the membrane plane and a key role of transmembrane electric currents in signal transduction (see below). In the eyespot region the plasma membrane and the outer chloroplast envelope are tightly connected, leaving, however, a free space with a constant width of 10 to 40 nm between them [17]. Electron microscopy studies revealed that this area of the plasma membrane is enriched with intramembranous particles of 8-12 nm diameter [22], which might represent complexes of receptor molecules. The outer chloroplast envelope has also been suggested as a plausible location of the photoreceptor molecules in green flagellates [23, 24]. However, this hypothesis seems to be less likely, since the photoreceptor current has virtually no lag-period [4].
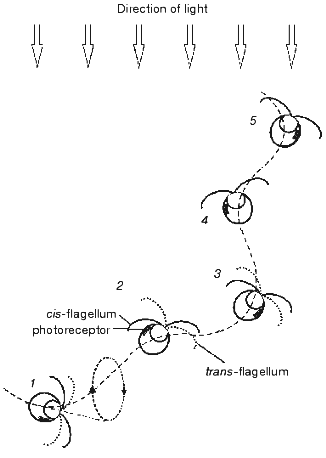
The amount of light incident on the photoreceptor periodically changes in the course of helical swimming, typical for most flagellates, when the axis of the helix is not coincident with the direction of the light. Light is absorbed by carotenoids accumulated in the eyespot lipid globules and by the chloroplast pigments, which causes shading of the photoreceptor when the eyespot faces away from the light source. Periodic alteration of the photoreceptor illumination is perceived by the cells as a signal to correct the swimming path. The mechanism of phototaxis is schematically presented in Fig. 1. The cell moves in a helical path that results from the rotation of the cell around its longitudinal axis and in the plane of flagellar beating. If the direction of the forward movement deviates from the light direction (1-3), the photoreceptor illumination changes during the rotation cycle. It reaches its maximum when the cell faces the light source with its eyespot (2), and minimum--when the cell is in the opposite phase of the cycle (1, 3). The difference in the photoreceptor illumination serves as the signal for the asymmetrical motor response of the two flagella, which takes place twice during one rotation cycle and leads to alignment of the swimming path with the light direction (1-3). When the swimming direction becomes parallel to the light direction as the result of corrective flagellar motions (4, 5), the photoreceptor illumination becomes constant during the rotation cycle, so that no more signal to change the swimming path is generated.
The importance of periodical shading of the photoreceptor for phototaxis was already recognized by early investigators [25, 26]. Later this notion has been complemented by a hypothesis that the eyespot in green flagellate algae, which consists of layers with different refractive indexes, acts as a quarter-wave stack enhancing the photoreceptor illumination when the eyespot faces the light source [16]. This mechanism should further improve directional sensitivity of the photoreceptor apparatus compared to that resulting from shading, since the interference reflection dramatically decreases when the angle of light incidence deviates from the normal to the eyespot surface.Fig. 1. Schematic representation of the mechanism for phototaxis in green flagellate algae: 1-5) consequent positions of the cell swimming under unilateral illumination with the actinic light. See further explanation in the text.
The efficiency of the modulation of the light signal during helical swimming was estimated by recording photoreceptor currents from a cell sucked into a pipette and illuminated at different angles with respect to the eyespot [18]. It has been shown that the amplitude of the photoreceptor current is much larger in the cell facing the light source with its eyespot, as compared to the cell in the opposite position. A weak dependence of the current amplitude on the angle that was observed within each of the two phases of illumination and shading of the photoreceptor points to a relatively small contribution of the interference mechanism to the modulation of the photoreceptor illumination in Haematococcus. This conclusion is consistent with the finding of only one layer of carotenoid globules in the eyespot of this microorganism [24]. In Chlamydomonas reinhardtii, which has the eyespot consisting of 2-4 layers of carotenoid globules [27], a shift measured between the stimulus-response curves for the photoreceptor current corresponds to approximately 8-fold attenuation of the photoreceptor illumination when the eyespot is turned away from the light source, as compared to the opposite position [28].
Some pigment-deficient Chlamydomonas mutants have neither eyespots, nor normal chloroplasts. Phototaxis observed in such mutants after incorporation of exogenous retinal has the opposite sign compared to the wild type. This is a direct consequence of their generation of maximal photoreceptor currents when being illuminated from the back side [10]. The most likely mechanism for this phenomenon is focusing of the light by the almost transparent cell on the photoreceptor membrane, i.e., “lens effect” [5].
SIGNAL TRANSDUCTION MECHANISMS
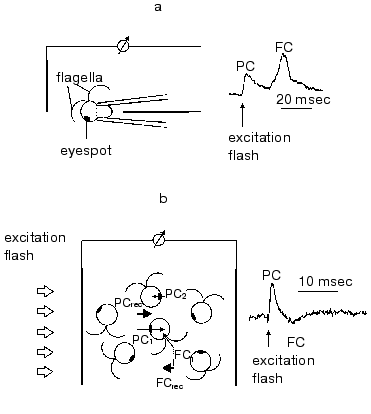
In green flagellate algae transduction of the light stimulus to the intracellular signal and its transmission to the flagellar apparatus occurs via generation of a cascade of rapid photoelectric responses of the cell membrane, which is triggered by photoexcitation of the receptor [29, 30]. The responses of this cascade were initially observed by extracellular recording with a suction pipette [7, 8]. A method for photoelectric recording in cell suspensions was developed later [31] (Fig. 2). Using traditional intracellular microelectrodes is impeded by the high sensitivity of these responses to a mechanical damage of the membrane [6], whereas the application of the patch-clamp technology has not so far succeeded, because a complete removal of the cell wall could not be achieved.
The involvement of the extracellularly recorded photoinduced currents in phototaxis is unambiguously confirmed by a long list of experimental evidences: spectral and light sensitivity, localization, inhibitory analysis, experiments with blind mutants, etc.Fig. 2. A scheme for measurement of photoinduced electrical signals involved in the signal transduction chain for phototaxis in green flagellate algae (PC, photoreceptor current; FC, flagellar current): a) signal recording in individual cells with a suction-pipette technique; b) signal recording in a suspension of non-oriented cells. The index 1 shows the currents from individual cells facing the light source with their photoreceptors; the index 2, those facing away from it; the index rec, resultant (recorded) currents.
The phototactically active light induces a constant flow of the current across a defined portion of the membrane in the eyespot region. After switching off the light this current decays to zero in ~50 msec, i.e., within characteristic time of the passive discharge of the membrane. At high stimulus intensities the current kinetics comprises an initial peak, the amplitude of which may exceed that of the subsequent stationary phase more than an order of magnitude [21, 29, 30]. The light saturation of the stationary level is at least an order of magnitude lower than that of the peak amplitude and almost corresponds to the light saturation of phototaxis.
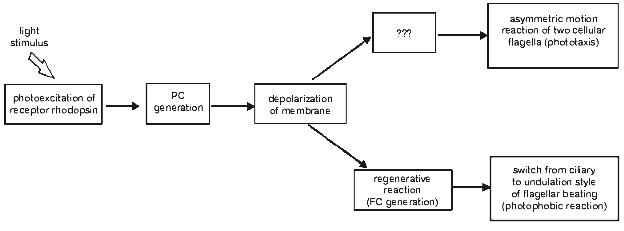
Optical monitoring of flagellar beating in a cell sucked into a micropipette revealed that a step-up light stimulus induced an increase in the beat frequency of the cis-flagellum (the one closest to the eyespot), and the decrease in the beat frequency of the trans-flagellum, whereas step-off stimulus caused the opposite responses [18, 29, 30, 32]. The observed beat frequency changes were accompanied by slight changes in beating curvature, also opposite in the two flagella of the cell. Such unbalanced motor responses of the two flagella would lead to the correction of the swimming path in a freely swimming cell, i.e., to phototaxis. Therefore, it could be concluded that gradual changes in the amplitude of the stationary photoreceptor current constitute the initial step in the signal transduction chain for phototaxis (Fig. 3).
When the stimulus intensity and/or duration exceeds a certain threshold, the photoreceptor current is superimposed by a transient signal of a complex waveform, initially termed as the “regenerative response” [7, 8]. This response appears as an “all-or-nothing” event, i.e., its amplitude only slightly depends on or does not change much upon variation of the stimulus intensity, whereas its lag period becomes shorter when the intensity increases. The sign of its major peak measured in a cell sucked into a pipette depends on whether the flagella are inside or outside the pipette, which shows that it reflects an inward transmembrane current in the flagellar-bearing region of the cell [29, 33]. Therefore, this current has been referred to as the “flagellar current” [33]. It is highly sensitive to the removal of Ca2+ ions from the extracellular medium. The integral under the photoreceptor current before the onset of the flagellar current is constant at any stimulus intensity, which indicates that the flagellar current likely reflects opening of voltage-gated Ca2+ channels [29, 30]. These channels appear to be localized to the membrane of flagella and evenly distributed along their whole length [34].Fig. 3. A scheme for the signal transduction chain for phototaxis and the photophobic response in green flagellate algae (PC, photoreceptor current; FC, flagellar current).
A close time correlation between the appearance of the regenerative response and a switch from ciliary style of flagellar beating to undulation shows that it is the basis for the photophobic response of the cell. This notion has been recently confirmed by simultaneous measurement of photoelectric currents and flagellar beating in the same cell [35] and by photoelectric measurements from suspensions of C. reinhardtii mutants deficient in the photophobic response [36, 37].
The onset of the photoreceptor current measured in single cells of Haematococcus pluvialis upon laser flash excitation is observed instantaneously within the time resolution of the measuring system (<30 µsec) [38]. However, analysis of the signal rise shows the presence of a current component with a lag period of several hundreds of microseconds, the duration of which depends on the stimulus intensity [38]. Switching on the red background illumination known to hyperpolarize the cell membrane [6], only increases the amplitude of the delayed component of the photoreceptor current [38]. The two components distinguishable in the current rise are referred to as the “early” and “late” photoreceptor currents [4, 29, 30]. The flash-to-peak time of the photoreceptor current shortens with the increase in the stimulus intensity [31]. Its decay can be deconvoluted by at least two exponential components, although they cannot be directly related to the components of the signal rise [38].
The presence of a light-dependent delay and the sensitivity of the current amplitude to the physiological state of the cell indicates the likely involvement of biochemical mechanisms in generation of the late photoreceptor current. Several enzymes characteristic for sensory transduction cascades in animals have been detected in isolated eyespot preparations of green flagellate algae (see below), although their possible role in phototaxis signaling has yet to be elucidated. On the contrary, the absence of a lag period for the early receptor current shows that it is maintained by the rhodopsin itself, or by a closely associated ion channel. Fast signals reflecting intramolecular processes are recorded, for instance, from purple membranes that contain bacteriorhodopsin [39]. An electrical signal with similar properties, also called the “early photoreceptor current”, is known in animal visual cells, where it can be directly correlated to the photochemical conversion of the visual rhodopsin monitored by optical methods [40, 41]. In contrast to the currents in animal visual cells, the early receptor current in green flagellate algae is significantly overlapped by the late receptor current. This feature and the present absence of spectroscopy data on algal photoreceptor pigments significantly complicate investigation of the early receptor current in flagellates. Therefore, it is difficult to estimate how similar is the origin of the early receptor current in green flagellate algae to that in animal visual cells, where several processes, such as chromophore dipole orientation, fixed charge separation, macrodipole (alpha helix) movement, intramolecular proton transfer, and vectorial proton uptake or release from the interfacial membrane boundary solutions, have been proposed to contribute to it [42, 43].
Measurement of the dependence of the peak amplitude of the photoreceptor current in green flagellates on the stimulus intensity also reveals its complex nature. It was already found in experiments in individual cells that an exponential saturation function can only be fitted to the data assuming a contribution of a second low-saturating process [29].
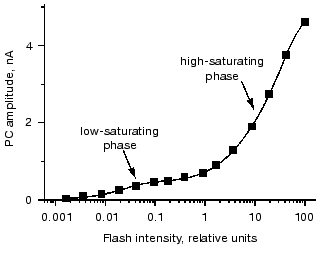
Two phases of the stimulus-response curve are most clearly seen when the currents are measured in cell suspensions, since in this case measurements can be extended to lower light intensities due to a high signal-to-noise ratio [31]. Fitting of the curve is only possible with two saturation functions (Fig. 4). The ratio between the amplitudes of the low- and high-saturating components varies from 1 : 10 to 1 : 5, and the ratio between their saturation levels varies from 1 : 300 to 1 : 50 in different species and culture states.
One possible explanation for the low light saturation of the first phase of the stimulus-response curve is its being limited by the concentration of a secondary messenger. Therefore, a hypothesis has been suggested that the low-saturating phase of the curve reflects saturation of the late photoreceptor current, whereas the high-saturating phase is determined by the saturation of the early photoreceptor current [4]. However, this hypothesis cannot explain the high sensitivity of the current amplitude to the physiological state of the cell at high stimulus intensities, so that the interpretation of the two phases of the stimulus-response curve at present remains unclear.Fig. 4. Dependence of the amplitude of the photoreceptor current (PC) recorded in suspensions of Chlamydomonas reinhardtii on the intensity of the excitation flash. Solid line is the result of a computer fit with a sum of two hyperbolic functions.
The causal relationship between the two receptor currents in green flagellate algae is also not yet known. Since most physiological evidences point to a single rhodopsin species to mediate both phototaxis and the photophobic response, it is likely that this species is responsible for both currents that develop in parallel or in series. However, a possibility of the two currents being mediated by different rhodopsins cannot be totally ruled out [44].
Molecular components of the enzymatic signaling cascade that is characteristic for visual receptor cells have been identified by biochemical methods in the eyespot preparations from green flagellate algae. The isolated Chlamydomonas eyespots have been reported to in vitro mediate the light-dependent activity of cGMP-phosphodiesterase from bovine retina in the presence of bovine transducin [45, 46]. Membranes of a carotenoid-deficient Chlamydomonas mutant did not have this capacity, unless they were preincubated with 11-cis- or all-trans-retinal [47]. The membranes, preincubated with either retinal isomer, but not untreated, also catalyzed photostimulation of transducin GTPase [47]. These findings have been interpreted as an indication to that Chlamydomonas phototaxis receptor has the binding site for animal transducin [46].
Several proteins with characteristics of subunits of heteromeric G proteins were detected in the eyespot preparations from the green flagellate algae C. reinhardtii and S. similis [48-50]. The GTPase activity measured in the isolated eyespots of S. similis is regulated by light and Ca2+ [51]. The antibodies raised against the retinal-binding protein identified in the eyespots of C. reinhardtii [52] recognized a specific 32 kD protein in the eyespot preparations of S. similis and inhibited light modulation of their GTPase activity [51]. In addition, Ca2+-dependent protein kinase and phosphatase activities were found in these preparations [50, 53].
These data led to a hypothesis that heteromeric GTPases are involved in the signal transduction chain in green flagellate algae, which is mostly supported by an observation that the light dependence and spectral sensitivity of the photoinduced inhibition of the GTPase activity correlate to those of phototaxis [51]. However, it is difficult to establish whether this correlation is specific due to a large amount of carotenoids and other pigments with spectral characteristics close to those of the receptor rhodopsin found in the eyespot preparations. Besides that, it is not known to what extent results obtained in isolated eyespot preparations reflect processes in intact cells rather than artifacts related to cell disruption.
THE PHOTOTAXIS RECEPTOR IS A RETINYLIDENE PROTEIN
Initial ideas on the chemical nature of receptors for photobehavioral responses were gained by measurement of their spectral sensitivity. Action spectra with maxima between 450 and 500 nm have been reported for phototaxis and the photophobic response in a number of Chlorophyta (Platymonas subcordiformis, Dunaliella salina, Stephanoptera gracilis,gametes of Ulva taeniata and Ulva rigida [54]; Chlamydomonas reinhardtii [55]; Volvox aureus [56]), as well as in the dynoflagellate Gymnodinium splendens [57]. Such spectra point to the pigments containing flavins or carotenoids as their chromophores. Lack of a near UV band in phototaxis spectra in the above microorganisms ruled out the former possibility [58]. Coincidence of the overall shape of these spectra and that of the absorption spectrum of animal visual rhodopsin led to the hypothesis that phototaxis receptors in green flagellate algae might be homologous to the pigments of animal vision [16].
Theoretical considerations imply that the action spectrum of phototaxis should result from a superposition of the absorption spectrum of the receptor pigment and the spectral characteristics of the eyespot and chloroplast, which modulate the photoreceptor illumination during phototaxis. This notion has been experimentally confirmed [56, 59, 60]. The action spectrum that provides the closest match of the absorption spectrum of the photoreceptor pigment was obtained by measurement of the photoreceptor current at maximal illumination of the photoreceptor [7, 8, 10]. Comparison of this action spectrum with that of phototaxis revealed that the contribution of the eyespot and chloroplast (except the long-wavelength area of the latter) did not significantly shift the phototaxis action spectrum from the absorption spectrum of the receptor pigment.
Investigation of the photoreceptor current, which is the earliest detectable event in the signal transduction chain of phototaxis, enabled the resolution of characteristics of the photoreceptor pigment that is not presently available for application of spectroscopic and biochemical methods. An estimate of the product of the optical cross section and quantum efficiency calculated from measuring the stimulus-response dependence of the peak amplitude of the photoreceptor current is approximately 8*10-21 m2, which is close to that of other known retinal proteins [29, 30]. Besides providing indirect evidence for the rhodopsin nature of the pigment, this result rules out the involvement of a light-harvesting (“antenna”) complex in the phototaxis receptor.
Maximal amplitude of the photoreceptor current was recorded when the plane of polarization of the light stimulus was parallel to the photoreceptor membrane [18, 30, 61]. This result shows that transition dipole moments of chromophore molecules lie in the plane of the cell membrane, which is typical for animal rhodopsins [62] and bacteriorhodopsin [63].
Specific inhibition of phototaxis and the photoreceptor current in green flagellate algae Chlamydomonas and Haematococcus by hydroxylamine, an agent known to induce light-dependent cleavage of the chromophore in retinal-containing proteins, i.e., those in which retinal binds to the apoprotein via formation of a Schiff base, further proved the rhodopsin nature of the phototaxis receptor [64, 65]. But, the most unambiguous evidence for such nature has been obtained by reconstitution studies in a carotenoid-deficient mutant of C. reinhardtii [9]. Such mutants are blind, i.e., their phototaxis sensitivity is several orders of magnitude below that of the wild type. However, it can be restored by the addition of exogenous retinal [9]. No photoinduced electrical currents could be detected in blind mutants unless they were reconstituted with retinal [10]. This indicated that restoration of their phototaxis sensitivity after the addition of retinal indeed resulted from reconstitution of the functional receptor from a normal apoprotein, expressed in these mutants, with the exogenous chromophore rather than from the influence of retinal on downstream elements of the signal transduction chain or its incorporation into the eyespot. These data strongly support the notion that the native phototaxis receptor in Chlamydomonas is a retinylidene protein.
C. reinhardtii is the only so far studied flagellate species where carotenoid-deficient mutants are available, which made it possible to carry out retinal reconstitution studies to directly prove the rhodopsin nature of the phototaxis receptor. Inhibition of phototaxis in Haematococcus lacustris observed when the cells were cultivated in the presence of the carotenoid biosynthesis inhibitor norflurazon [66], does not necessarily reflects the loss of the functional photoreceptor, but might be explained by deterioration of the eyespot by this treatment. However, the involvement of rhodopsin-type receptors in phototaxis and the photophobic response can at present be postulated for a much wider range of flagellate species, in the first place those that possess intrachloroplast eyespots. This view is strongly supported by measurement of typical photoreceptor currents in a number of flagellatealgae, including Chlamydomonas, Haematococcus, Polytomella, Spermatozopsis, Hafniomonas [17, 67] and Volvox [21], i.e., in most of the objects with rhodopsin-like phototaxis action spectra.
Two major groups of so far known retinal proteins comprise animal visual rhodopsins and proteins responsible for photoreception and energy conversion in archaea [68]. Is is noteworthy that there is practically no homology between primary sequences of the proteins that belong to the different groups. Besides this, the proteins from the two groups contain different retinal isomers as chromophores and undergo different primary reactions upon photoexcitation. In archaeal rhodopsins all-trans-retinal chromophore photoisomerizes into 13-cis-, whereas 11-cis-retinal is the most frequently found chromophore in animal visual rhodopsins, which converts into all-trans-retinal during the photocycle. A recent finding of a archaeal-type rhodopsin in fungi [69, 70] and eubacteria [71] shows that proteins of this type are widely spread among representatives of evolutionarily very distant taxa. Taking this into account, identification of what particular retinal isomer serves as the chromophore in phototaxis receptors in green flagellate algae appears to be extremely important for characterization of these pigments.
CHROMOPHORE PROPERTIES
Investigation of the recovery of phototactic ability in blind Chlamydomonas mutants upon the addition of exogenous compounds [9] turned out to be an excellent experimental system for elucidation of chromophore requirements of algal rhodopsins. However, use of this assay resulted in a major controversy between the results of initial experiments carried out in the FN68 strain of C. reinhardtii by measuring the light-induced migration of a cell population in a Petri dish [73, 74] and subsequent studies that employed more technically advanced methods.
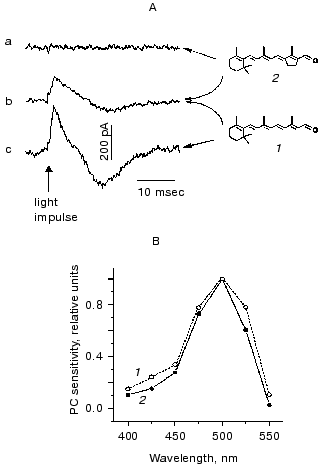
Initial conclusions drawn from measurements of the population migration were that the photoreceptor in Chlamydomonas incorporates 11-cis-retinal and is homologous to animal rhodopsins [9]. Furthermore, it was also concluded that its photoactivation does not involve cis-trans-isomerization of the chromophore [72-74]. However, these notions could not be confirmed by video recording and motion analysis of individual cell tracks in the CC2359 blind Chlamydomonas strain [75, 76]. Neither phototaxis, nor the photophobic response assayed by this method could be restored by the addition of retinal analogs prevented from isomerization around the C13=C14 double bond (locked in either the 13-trans- or 13-cis-configuration). Moreover, these analogs were inefficient in restoration of photoreceptor currents in blind mutants, although entered the retinal binding site, as it could be concluded from their inhibition of the response induced by all-trans-retinal [10] (Fig. 5). Furthermore, it was all-trans-retinal which induced most rapid and most complete reconstitution of both phototaxis and the photophobic response compared to its cis-isomers [75, 76]. Similar results were obtained when photobehavioral responses were monitored by recording light scattering transients in cell suspensions [77].
Taking into account all these observations, it was concluded that all-trans-retinal is the native chromophore of Chlamydomonas rhodopsin(s) responsible for both phototaxis and the photophobic response [78, 79]. Photoexcitation of the receptor gives rise to isomerization of the chromophore to a 13-cis-form, as it takes place in all so far known archaeal-type rhodopsins. Common features of the chromophore of Chlamydomonas photoreceptor and those of archaeal rhodopsins revealed by reconstitution studies in blind mutants also include its 6-s-trans-conformation [76, 80]. Analysis of the efficiency of various ring/chain conformers in restoration of the photophobic response in Chlamydomonas reinhardtii and Halobacterium salinarium, and the results of the optical studies on reconstituted archaeal rhodopsins led to the conclusion that the retinal binding site of Chlamydomonas photoreceptor is phylogenetically related to that of the sensory rhodopsin II [80]. The functional chromophore of Chlamydomonas rhodopsin requires the presence of at least three conjugated C=C double bonds in the polyene chain and a methyl group at C13 position, as it has been found by a behavioral assay [77] and by recording photoelectric currents in cell suspensions [10].Fig. 5. A) Photoelectric signals measured in suspensions of the carotenoid-deficient strain CC2359 of Chlamydomonas reinhardtii after the addition of retinal (1), its 13-trans-locked analog (2) or their combination: a) cells were preincubated with 15 nM analog; b) the same, but 10 nM retinal was added afterwards; c) cells were preincubated with 10 nM retinal. B) Action spectra for the photoreceptor current measured in suspensions of a carotenoid-deficient mutant after the addition of 5 nM retinal (1) and the wild type of Chlamydomonas reinhardtii (2).
The discrepancies between the results of reconstitution experiments in “blind” Chlamydomonas cells reported by Foster's group and those of subsequent studies might have several reasons. These are using in the former case high chromophore concentrations that might contain trace retinal impurities sufficient to restore the photoreceptor function, and that might accumulate in the eyespot lipid globules [81], and also using extended (10 min) illumination for measurement of the population migration, which might be enough to induce the synthesis of endogenous retinal [82].
In vitro approaches to identification of the chromophore of algal rhodopsins included high performance liquid chromatography (HPLC) analysis of organic extracts and optical spectroscopy studies on isolated photoreceptor preparations. Differential centrifugation of subcellular material obtained by disruption of cells allows separation of the eyespot fraction. This fraction can be recognized as a highly pigmented orange-colored band due to the presence of carotenoid globules [83, 84]. Electron microscopy revealed that the isolated eyespots retain the leaflet of the plasma membrane where the photoreceptor molecules are presumably located [84]. In Chlamydomonas, all-trans-retinal was the major isomer detected in such preparations, although a small amount of 13-cis-, but not 11-cis-, retinal was also found [83, 85]. However, both all-trans- and 11-cis-isomers were extracted from the eyespot preparations of Spermatozopsis [86].
As it has been already mentioned, phototactic sensitivity in the carotenoid-deficient FN68 Chlamydomonas strain can be restored by irradiation with green light, which presumably activated retinal biosynthesis [82]. The amount of all-trans-retinal extractable from these cells during the irradiation increased in parallel with the increase in phototactic sensitivity, and reached ~30,000 molecules per cell [83], which is close to an estimate of the number of receptor molecules calculated from the threshold sensitivity of phototaxis [16]. This observation indicates that the extractable all-trans-retinal serves as the chromophore of the phototaxis receptor.
Detection of the rhodopsin by absorption spectroscopy in isolated eyespots is very much compromised by both high content of carotenoids still found in these preparations even after detergent treatment, and by their intense light scattering due to the presence of membrane fragments. Absorption changes observed after bleaching of the isolated eyespots with green light seem to be mostly driven by oxidation of carotenoids, since it was significantly reduced in the presence of the antioxidant alpha-tocopherol (E. Govorunova, unpublished observations). The very little effect of hydroxylamine on the bleaching process [83] also supports this view. The addition of exogenous retinal has been reported to restore the absorption of the bleached eyespot preparations, although different retinal isomers were found to be the most efficient in different studies [83, 86, 87]. This discrepancy likely indicates that the enrichment of the receptor content in the eyespots preparations is insufficient for the application of optical spectroscopy methods.
THE SEARCH FOR OPSIN GENES
All-trans-retinal chromophore of phototaxis receptors of green flagellate algae points to their similarity with archaeal rhodopsins. However, all attempts to identify genes homologous to those of archaeal rhodopsins which have been undertaken by several research groups, were, to the best of our knowledge, so far unsuccessful.
On the other hand, a homologous fragment was detected in DNA isolated from Chlamydomonas using a bovine rhodopsin complementary DNA probe for hybridization [88]. However, this result does not necessarily imply that this fragment encodes the phototaxis receptor protein rather than any other G-protein-coupled membrane receptor which might also be present in unicellular algae.
Insertional mutagenesis has been used to identify genes that encode molecular components of the phototaxis signaling pathway in Chlamydomonas, and 12 ptx mutations that cause defects in phototaxis were identified by this approach [36]. However, most of these mutants displayed normal photoreceptor currents, thus bearing a deficiency in the signaling cascade downstream from the photoreceptor. In those cases when inhibition of the photoreceptor current was observed, its detailed investigation revealed that this inhibition was not due to disruption of the receptor protein, but rather due to indirect reasons. In particular, a decreased amplitude of the photoreceptor current recorded by a population assay in the ptx4 mutant is the consequence of its multiple eyespots. A twofold reduction of the photoreceptor current in the ptx3 mutant is not enough to explain its large defect in phototaxis, so that the current appears to be indirectly affected by this mutation. Therefore, no mutants that have the phenotype expected for the gene encoding the photoreceptor protein have been so far obtained by this approach [36].
An alternative strategy for identification of a phototaxis receptor gene assumed a protein as the starting point. A single protein was detected by labeling with 3H-containing retinal in the eyespot membrane fraction from the wild type Chlamydomonas cells, which constituted about 25% of total protein in this preparation, but was almost absent in other cell membranes [83]. Preincubation of the membranes with 9-azidoretinal, which, according to the results of physiological reconstitution studies, cannot enter the retinal binding site due to its bulky substituent, improved labeling with 3H-containing retinal likely due to saturation of non-specific binding of the chromophore [52]. This protein has been purified to homogeneity by SDS-PAGE and used to raise a polyclonal antibody [11]. Immunofluorescence experiments have shown that the antibody concentrates in a small clearly defined area recognized as the eyespot in partially permeabilized cells, although direct observation of the eyespot position in such cells was not possible [11].
Peptides derived from the purified protein were used for mRNA isolation and preparation of cDNA that contained an open reading frame encoding a protein of 235 amino acids [11]. This amino acid sequence, referred to as chlamyopsin, shows some homology to invertebrate opsins, but not to the opsins from archaea, and contains many polar and charged residues, as is typical for ion channels [11]. The chlamyopsin gene could be heterologously expressed with a high yield in Escherichia coli, Schizosaccharomyces pombe, and Pichia pastoris, but no retinal binding was observed in the resulting protein (W. Deininger, unpublished observations). A synthetic codon-adapted gene encoding green fluorescent protein (GFP) was fused to the chlamyopsin gene and expressed in C. reinhardtii [89]. Although the expression level of the chlamyopsin-GFP was low, fluorescence microscopy studies have shown that the fusion protein was localized to the eyespot region of the cell [89].
Screening of the cDNA library of the colonial alga Volvox carteri with fragments of the chlamyopsin cDNA resulted in identification of a clone carrying a cDNA insert encoding a protein with the deduced amino acid sequence 61% identical to that of chlamyopsin [12]. This protein, named volvoxopsin, was expressed in S. pombe, and the antibodies raised against it were used to study its expression during the lifecycle of Volvox. Surprisingly, the volvoxopsin concentration was found to be higher in the total membrane fraction of gonidia, which lack visible eyespots, than in the total membrane fraction of somatic cells [12].
All attempts to produce knockout mutants for opsin genes either in Chlamydomonas or Volvox were so far unsuccessful, which significantly complicates functional testing of the products of these genes as possible candidates for phototaxis receptors. However, in both microorganisms, reduction of the opsin concentration could be achieved by transformation with antisense constructs [12, 13]. In Volvox, a decreased light sensitivity of photoaccumulation in the opsin antisense transformant was reported [12]. However, the analysis of the opsin antisense transformants produced from three different strains of Chlamydomonas revealed no difference in the light sensitivity of their photoreceptor currents, phototactic orientation and the photophobic response, as compared to their parental strains [13]. Therefore, the functional role of the product of this gene identified in Chlamydomonas remains unclear, but its being the phototaxis receptor seems to be unlikely.
Today we can state that substantial efforts invested in studies on phototaxis in green flagellate algae since the discovery of this phenomenon at the end of the XIX century succeeded in a rather clear outline of the orientation mechanism and basic components of the signaling cascade. There are also no doubts that rhodopsin-type proteins serve as phototaxis receptors in this group of microorganisms, although their unambiguous identification has not been achieved yet. Their properties revealed by indirect methods indicate that these pigments constitute a unique group of retinal-binding proteins.
On one hand, they share the same chromophore type with archaeal rhodopsins. Identification of genes, homologous to those of archaeal rhodopsins, in some eukaryotic microorganisms shows a possibility of the presence of such genes also in green flagellate algae, although they have not yet been found there. On the other hand, the involvement of photoelectric processes in the signal transduction chain is a common feature of phototaxis receptors and animal visual rhodopsins. The presence of several components of the visual enzymatic cascade in the eyespot preparations further supports the similarity between algal and animal signaling systems. At the same time, the photoreceptor function of the retinal-binding proteins found in Chlamydomonas and Volvox, whose primary sequences show a homology to animal rhodopsins, at present cannot be considered proven.
Therefore, it can be concluded that further investigation of the properties of the unique rhodopsin receptors for phototaxis in green flagellate algae and, in the first place, identification of the genes encoding for them, will likely lead to a substantial extension of our image of the whole superfamily of retinal-containing proteins, among which only two major groups have been recognized so far.
The authors thank Prof. F. F. Litvin for his help and interest in this work.
The work was supported by the Russian Foundation for Basic Research (project No. 99-04-49015).
REFERENCES
1.Diehn, B., Feinleib, M., Haupt, W., Hildebrand, E.,
Lenci, F., and Nultsch, W. (1977) Photochem. Photobiol.,
26, 559-560.
2.Sineshchekov, O. A., and Litvin, F. F. (1974)
Uspekhi Sovrem. Biol., 78, 58-75.
3.Sineshchekov, O. A., and Litvin, F. F. (1982)
Uspekhi Mikrobiol., 17, 62-87.
4.Sineshchekov, O. A., and Govorunova, E. G. (1999)
Trends Plant Sci., 4, 58-63.
5.Sineshchekov, O. A., and Govorunova, E. G. (2001)in
Comprehensive Series in Photosciences,Vol. 1,
Photomovements (Haeder, D.-P., Lebert, and Jori, G., eds.) Elsevier
Science, pp. 245-280.
6.Sineshchekov, O. A., Andrianov, V. K., Kurella, G.
A., and Litvin, F. F. (1976) Fiziol. Rast., 23,
229-237.
7.Sineshchekov, O. A., Sineshchekov, V. A., and
Litvin, F. F. (1978) Doklady AN SSSR, 239,
471-474.
8.Litvin, F. F., Sineshchekov, O. A., and
Sineshchekov, V. A. (1978) Nature, 271, 476-478.
9.Foster, K.-W., Saranak, J., Patel, N., Zarrilli,
G., Okabe, M., Kline, T., and Nakanishi, K. (1984) Nature,
311, 756-759.
10.Sineshchekov, O. A., Govorunova, E. G., Der, A.,
Keszthelyi, L., and Nultsch, W. (1994) Biophys. J., 66,
2073-2084.
11.Deininger, W., Kroeger, P., Hegemann, U.,
Lottspeich, F., and Hegemann, P. (1995) EMBO J., 14,
5849-5858.
12.Ebnet, E., Fischer, M., Deininger, W., and
Hegemann, P. (1999) Plant Cell, 11, 1473-1484.
13.Fuhrmann, M., Stahlberg, A., Govorunova, E.,
Rank, S., and Hegemann, P. (2001) J. Cell Sci., 114,
3857-3863.
14.Rueffer, U., and Nultsch, W. (1985) Cell
Motil. Cytoskeleton, 5, 251-263.
15.Melkonian, M., and Robenek, H. (1984) Prog.
Phycol. Res., 3, 193-268.
16.Foster, K.-W., and Smyth, R. D. (1980)
Microbiol. Rev., 44, 572-630.
17.Kreimer, G. (1994) Int. Rev. Cytol.,
148, 229-310.
18.Sineshchekov, O. A. (1988) in Phototrophic
Microorganisms (Gogotov, I. N., ed.) [in Russian], AN SSSR,
Pushchino, pp. 11-18.
19.Ristori, T., Ascoli, C., Banchetti, R., Parrini,
P., and Petracchi, D. (1981) in Proc. 6th Int. Congr.
Protozool., Warsaw, p. 314.
20.Holland, E.-M., Braun, F.-J., Nonnengaesser, C.,
Harz, H., and Hegemann, P. (1996) Biophys. J., 70,
924-931.
21.Braun, F.-J., and Hegemann, P. (1999) Biophys.
J., 76, 1668-1678.
22.Melkonian, M., and Robenek, H. (1980) J.
Ultrastruct. Res., 72, 90-102.
23.Melkonian, M., and Robenek, H. (1979)
Protoplasma, 100, 183-197.
24.Ristori, T., and Rosati, G. (1983) Monit.
Zool. Ital., 17, 401-408.
25.Buder, J. (1917) Jahrb. wiss. Bot.,
58, 105-220.
26.Mast, S. O. (1927) Arch.
Protistenkd.,60, 197-220.
27.Sager, R., and Palade, G. E. (1954) Exp. Cell
Res., 7, 584-588.
28.Harz, H., Nonnengaesser, C., and Hegemann, P.
(1992) Phil. Trans. R. Soc. Lond. B, 338, 39-52.
29.Sineshchekov, O. A. (1991) in Light in Biology
and Medicine (Douglas, R. D., ed.)Vol. 2, Plenum Press, N. Y., pp.
523-532.
30.Sineshchekov, O. A. (1991) in Biophysics of
Photoreceptors and Photomovements in Microorganisms (Lenci, F.,
Ghetti, F., Colombetti, G., Haeder, D.-P. , and Song, P.-S., eds.)
Plenum Press, N. Y., pp. 191-202.
31.Sineshchekov, O. A., Govorunova, E. G., Der, A.,
Keszthelyi, L., and Nultsch, W. (1992) J. Photochem. Photobiol. B:
Biol., 13, 119-134.
32.Sineshchekov, O. A. (1983) in Application of
Lasers in Biology [in Russian], Moscow State University, Moscow,
pp. 91-98.
33.Harz, H., and Hegemann, P. (1991) Nature,
351, 489-491.
34.Beck, C., and Uhl, R. (1994) J. Cell
Biol., 125, 1119-1125.
35.Holland, E.-M., Harz, H., Uhl, R., and Hegemann,
P. (1997) Biophys. J., 73, 1395-1401.
36.Pazour, G. J., Sineshchekov, O. A., and Witman,
G. B. (1995) J. Cell Biol., 131, 427-440.
37.Matsuda, A., Yoshimura, K., Sineshchekov, O. A.,
Hirono, M., and Kamiya, R. (1998) Cell Mot. Cytoskeleton,
41, 353-362.
38.Sineshchekov, O. A., Litvin, F. F., and
Keszthelyi, L. (1990) Biophys. J., 57, 33-39.
39.Drachev, L. A., Kaulen, A. D., and Skulachev, V.
P. (1978) FEBS Lett., 87, 161-167.
40.Cone, R. A., and Pak, W. L. (1971) in Handbook
of Sensory Physiology, Vol. I, Springer-Verlag, Berlin, pp.
345-365.
41.Trissl, H.-W. (1982) Meth. Enzymol.,
81, 431-439.
42.Sullivan, J. M., and Shukla, P. (1999)
Biophys. J., 77, 1333-1357.
43.Sullivan, J. M., Brueggemann, L., and Shukla, P.
(2000) Meth. Enzymol., 315, 268-293.
44.Govorunova, E. G., and Sineshchekov, O. A.
(2001)in Recent Res. Devel. Plant Physiol. (Pandalai, S. G.,
ed.) Vol. 2, Research Signpost, Trivandrum, pp. 79-93.
45.Dumler, I. L., Korolkov, S. N., Garnovskaya, M.
N., Parfenova, D. V., and Etingof, R. N. (1989) J. Protein
Chem., 8, 387-389.
46.Korolkov, S. N., Garnovskaya, M. N., Basov, A.
S., and Dumler, I. L. (1989) Zh. Evolyuts. Biokhim. Fiziol.,
25, 777-780.
47.Korolkov, S. N., and Etingof, R. N. (1994)
Biol. Membr. (Moscow), 11, 161-168.
48.Korolkov, S. N., Garnovskaya, M. N., Basov, A.
S., Chunaev, A. S., and Dumler, I. L. (1990) FEBS Lett.,
270, 132-134.
49.Hegemann, P., and Harz, H. (1993) in Signal
Transduction: Prokaryotic and Simple Eukaryotic Systems, Academic
Press, San Diego, pp. 279-307.
50.Schlicher, U., Linden, L., Calenberg, M., and
Kreimer, G. (1995) Eur. J. Phycol.,30, 319-330.
51.Calenberg, M., Brohnsonn, U., Zedlacher, M., and
Kreimer, G. (1998) Plant Cell, 10, 91-103.
52.Kroeger, P., and Hegemann, P. (1994) FEBS
Lett., 341, 5-9.
53.Linden, L., and Kreimer, G. (1995) Planta,
197, 343-351.
54.Halldal, P. (1958) Physiol. Plantarum,
11, 118-153.
55.Nultsch, W., Throm, G., and von Rimscha, I.
(1971) Arch. Microbiol., 80, 351-369.
56.Schletz, K. (1976) Z. Pflanzenphysiol.,
77, 189-211.
57.Forward, R. B. (1974) J. Protozool.,
21, 312-315.
58.Halldal, P. (1961) Physiol. Plantarum,
14, 133-139.
59.Sineshchekov, O. A., Govorunova, E. G., and
Litvin, F. F. (1989) Biofizika, 34, 255-258.
60.Schaller, K., and Uhl, R. (1997) Biophys.
J., 73, 1573-1578.
61.Yoshimura, K. (1994) Photochem.
Photobiol., 60, 594-597.
62.Wald, G., Braun, P. K., and Gibbons, I. R. (1963)
J. Opt. Soc. Am., 53, 20-35.
63.Heyn, M. P., Cherry, R. J., and Mueller, U.
(1977) J. Mol. Biol., 117, 607-620.
64.Hegemann, P., Hegemann, U., and Foster, K.-W.
(1988) Photochem. Photobiol., 48, 123-128.
65.Sineshchekov, O. A., Govorunova, E. G., and
Litvin, F. F. (1991) Sensornye Sistemy, 5,
51-55.
66.Braune, W., and Ekelund, N. G. A. (1990) Arch.
Microbiol., 154, 448-452.
67.Sineshchekov, O. A., and Nultsch, W. (1992)
Proc. Vth Int. Conf. Retinal Proteins, Dourdan.
68.Spudich, J. L., Yang, C. S., Jung, K. H., and
Spudich, E. N. (2000) Annu. Rev. Cell. Dev. Biol., 16,
365-392.
69.Bieszke, J. A., Braun, E. L., Bean, L. E., Kang,
S., Natvig, D. O., and Borkovich, K. A. (1999) Proc. Natl. Acad.
Sci. USA, 96, 8034-8039.
70.Bieszke, J. A., Spudich, E. N., Scott, K. L.,
Borkovich, K. A., and Spudich, J. L. (1999) Biochemistry,
38, 14138-14145.
71.Beja, O., Aravind, L., Koonin, E. V., Suzuki, M.
T., Hadd, A., Nguyen, L. P., Jovanovich, S., Gates, C. M., Feldman, R.
A., Spudich, J. L., Spudich, E. N., and DeLong, E. F. (2000)
Science, 289, 1902-1906.
72.Foster, K.-W., Saranak, J., and Dowben, P. A.
(1991) J. Photochem. Photobiol. B: Biol., 8, 385-408.
73.Foster, K.-W., and Saranak, J. (1988) J. Am.
Chem. Soc., 110, 6589-6591.
74.Foster, K.-W., Saranak, J., Derguini, F.,
Zarrilli, G., Johnson, R., Okabe, M., and Nakanishi, K. (1989)
Biochemistry, 28, 819-824.
75.Takahashi, T., Yoshihara, K., Watanabe, M.,
Kubota, M., Johnson, R., Derguini, F., and Nakanishi, K. (1991)
Biochem. Biophys. Res. Commun., 178, 1273-1279.
76.Lawson, M. A., Zacks, D. N., Derguini, F.,
Nakanishi, K., and Spudich, J. L. (1991) Biophys. J., 60,
1490-1498.
77.Hegemann, P., Gaertner, W., and Uhl, R. (1991)
Biophys. J., 60, 1477-1489.
78.Spudich, J. L., Zacks, D. N., and Bogomolni, R.
A. (1995) Israel J. Chem., 35, 495-513.
79.Hegemann, P. (1997) Planta, 203,
265-274.
80.Sakamoto, M., Wada, A., Akai, A., Ito, M.,
Goshima, T., and Takahashi, T. (1998) FEBS Lett., 434,
335-338.
81.Kreimer, G., Overlaender, C., Sineshchekov, O.
A., Stolzis, H., Nultsch, W., and Melkonian, M. (1992) Planta,
188, 513-521.
82.Foster, K.-W., Saranak, J., and Zarrilli, G.
(1988) Proc. Natl. Acad. Sci. USA, 85, 6379-6383.
83.Beckmann, M., and Hegemann, P. (1991)
Biochemistry, 30, 3692-3697.
84.Kreimer, G., Brohnson, U., and Melkonian, M.
(1991) Eur. J. Cell Biol., 55, 318-327.
85.Derguini, F., Mazur, P., Nakanishi, K., Starace,
D., Saranak, J., and Foster, K.-W. (1991) Photochem. Photobiol.,
54, 1017-1021.
86.Kreimer, G., Marner, F.-J., Brohson, U., and
Melkonian, M. (1991) FEBS Lett., 293, 49-52.
87.Starace, D., and Foster, K.-W. (1989) Biophys.
J., 55, 379a.
88.Martin, R. L. C., Wood, C., Baehr, W., and
Applebury, M. L. (1986) Science, 232, 1266-1269.
89.Fuhrmann, M., Oertel, W., and Hegemann, P. (1999)
Plant J., 19, 353-361.