REVIEW: A Re-evaluation of the Antioxidant Activity of Purified Carnosine
E. A. Decker*, S. A. Livisay, and S. Zhou
Department of Food Science, Chenoweth Lab, University of Massachusetts, Amherst, MA 01003, USA; fax: 413-545-1262; E-mail: edecker@foodsci.umass.edu* To whom correspondence should be addressed.
Received December 22, 1999
The antioxidant activity of carnosine has been re-evaluated due to the presence of contaminating hydrazine in commercial carnosine preparations. Purified carnosine is capable of scavenging peroxyl radicals. Inhibition of the oxidation of phosphatidylcholine liposomes by purified carnosine is greater in the presence of copper than iron, a phenomenon likely to be due to the copper chelating properties of carnosine. Purified carnosine is capable of forming adducts with aldehydic lipid oxidation products. Adduct formation is greatest for alpha,beta-monounsaturated followed by polyunsaturated and saturated aldehydes. While the ability of carnosine to form adducts with aldehydic lipid oxidation products is lower than other compounds such as glutathione, the higher concentrations of carnosine in skeletal muscle are likely to make it the most important molecule that forms aldehyde adducts. Monitoring changes in carnosine concentrations in oxidizing skeletal muscle shows that carnosine oxidation does not occur until the later stages of oxidation suggesting that carnosine may not be as effective free radical scavenger in vivoas other antioxidants like alpha-tocopherol.
KEY WORDS: carnosine, antioxidant activity, alpha,beta-monounsaturated aldehydes
Carnosine and related dipeptides have been have been postulated to have numerous biological roles including pH buffering, regulation of enzyme activity, and inhibition of oxidative reactions. Among antioxidant mechanisms reported for carnosine are its ability to inactivate reactive oxygen species, scavenge free radicals, and chelate prooxidative metals [1-4]. A rather unusual reported antioxidant property of carnosine was its ability to reduced concentrations of thiobarbituric acid reactive substances (TBARS) when added to previously oxidized lipids [1, 4]. A potential mechanism for this observation would be the ability of carnosine to interact with aldehydic lipid oxidation products through Schiff base or Michael addition-type reactions. If carnosine could interact with aldehydic lipid oxidation products, this could potentially help protect biological tissues from oxidation, since aldehydes can form adducts with DNA, proteins, enzymes, and lipoproteins, causing alterations in their biological activity.
DISCOVERY OF CONTAMINATING HYDRAZINE IN SYNTHETIC SOURCES OF
CARNOSINE AND ANSERINE
Studies on the potential of carnosine to form adducts with aldehydic lipid oxidation products were conducted by reacting carnosine with saturated and alpha,beta-monounsaturated aldehydes and subsequent determination of alterations in aldehyde concentrations by gas chromatographic analysis of methylene chloride extracts and headspace gases as well as of alterations in carnosine concentrations by HPLC. Initial studies showed that synthetic carnosine rapidly caused a dramatic decrease in both saturated (hexanal) and alpha,beta-monounsaturated (trans-2-hexenal) concentrations [5]. Synthetic carnosine (25 mM) decreased hexanal concentrations 58% at pH 5.8 however, analysis of carnosine by HPLC under these same conditions did not show any decrease in carnosine concentrations. Carnosine was even more efficient at decreasing the concentrations of alpha,beta-monounsaturated aldehydes with trans-2-hexenal concentrations decreasing 82% under the same conditions. However, unlike hexanal, interactions between carnosine and trans-2-hexenal resulted in a decrease in carnosine concentration by 27%. The observed reduction of hexanal by synthetic carnosine without alterations in carnosine concentrations suggested that it was not carnosine that was interacting with hexanal.
Detailed analysis of gas chromatographs of methylene chloride extracts of synthetic carnosine-hexanal mixtures showed a large unidentified peak that did not appear in the chromatographs of carnosine or hexanal alone. Further investigation of this peak by mass spectroscopy, NMR, and elemental analysis revealed that the compound consisted of hydrazine adducted to two hexanals [5]. Subsequent analysis of synthetic sources of carnosine revealed that hydrazine was a common contaminant at concentrations ranging from 0.01 to 0.2%. Hydrazine was also found in synthetic anserine but not in dipeptides such as homocarnosine, alanyl-histidine, and glycyl-histidine. Since hydrazine was able to form adducts with hexanal, we determined whether it could also react with malonaldehyde, thus helping to explain the ability of synthetic carnosine to cause reductions in TBARS in previously oxidized lipids. Incubation of synthetic carnosine (0-25 mM) and hydrazine (at concentrations equivalent to the hydrazine concentrations in the synthetic carnosine) with malonaldehyde resulted in a 14-83% reduction in TBARS. In addition, both synthetic carnosine and hydrazine were able to reduce TBARS when added to previously oxidized phosphatidylcholine liposomes. In both cases, hydrazine was found to be primarily responsible for the ability of synthetic carnosine to decrease TBARS.
RE-EVALUATION OF THE ANTIOXIDANT ACTIVITY OF PURIFIED
CARNOSINE
Hydrazine is a powerful reducing agent that can inactivate free radicals, accelerate metal-promoted decomposition of lipid peroxides, and directly catalyze the reduction of peroxides to keto dienes. The presence of hydrazine in synthetic sources of carnosine places much of the published data on the antioxidant activity of synthetic carnosine in question since the contaminating hydrazine may be directly inhibiting lipid oxidation or may be interfering with lipid oxidation analysis. We have recently re-evaluated the antioxidant activity of purified carnosine to better understand the role of this dipeptide in oxidative reactions. Carnosine was separated from hydrazine by taking advantage of the fact that hydrazine readily forms adducts with hexanal while carnosine is left unaffected. Synthetic carnosine (80 ml of 50 mM solution) was reacted with 5 µl of hexanal for 20 min at 40°C. The resulting hydrazine-hexanal adduct, as well as unreacted hexanal, was removed by extraction with 30 ml of methylene chloride, which was repeated three times. The resulting carnosine had less than 2·10-4 wt. % hydrazine, a 50-fold reduction in hydrazine. The hydrazine concentration in the purified carnosine was not found to influence any subsequent analysis of the antioxidant activity of carnosine.
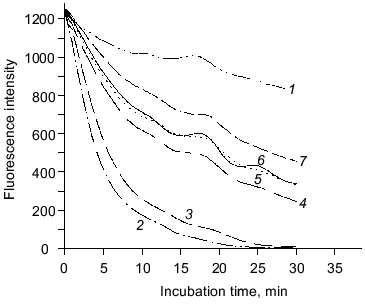
The ability of purified carnosine to scavenge peroxyl radicals has been evaluated in a model where peroxyl radicals are generated by 2,2´-azobis-(2-amidopropane) (AAPH) with peroxyl radical concentrations being determined using fluorometric analysis of beta-phycoerythrin oxidation [6]. Carnosine and homocarnosine (0.25 mM) had similar abilities to inactivate peroxyl radicals (Fig. 1). Histidine had slightly lower peroxyl radical scavenging activity than carnosine and homocarnosine, while leucyl-histidine had slightly greater activity. beta-Alanine exhibited very little peroxyl radical scavenging activity. These results show that purified carnosine is capable of scavenging peroxyl radicals, and the observation that beta-alanine does not inactivate peroxyl radicals while histidine does suggests that this radical scavenging activity is largely due to histidine.
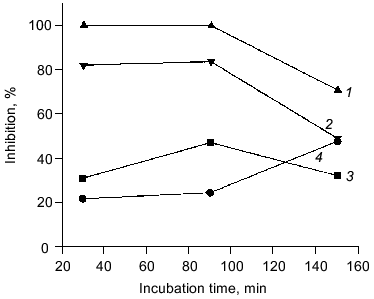
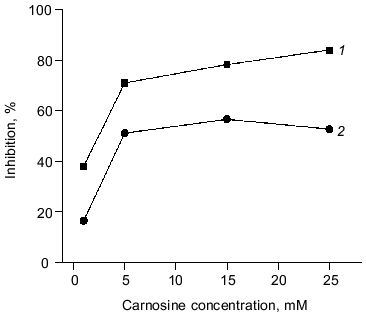
The antioxidant activity of purified carnosine was also evaluated in a phosphatidylcholine liposome (0.2 ml phospholipid/ml assay) model where oxidation was promoted by an ascorbate (100 µM)-metal redox cycling system using either Fe3+ (15 µM) or Cu2+ (30 µM). Carnosine was observed to have greater antioxidant activity when oxidation was promoted by copper rather than by iron as determined by both TBARS (Fig. 2) and headspace hexanal or pentane (Fig. 3) analysis. Histidine was also observed to inhibit headspace hexanal formation with its activity being lower than that of carnosine (26 versus 48% inhibition after 90 min of oxidation) in the presence of iron and being superior to carnosine (99 versus 83% inhibition after 90 min of oxidation) in the presence of copper. The increased antioxidant activity of carnosine and histidine when copper is used to promote oxidation is likely due to their ability to chelate and inactivate copper [2, 3]. Carnosine has not been found to chelate iron in a manner that reduces its prooxidant activity [3]. Therefore, the ability of carnosine to inhibit iron-promoted oxidation is likely due to free radical scavenging activity, whereas the antioxidant activity of carnosine in the presence of copper is likely due to a combination of chelation and free radical scavenging. Since the free radical scavenging activity of carnosine is greater than that of histidine (Fig. 1), this could explain why carnosine inhibits iron-promoted oxidation more effectively.Fig. 1. The ability of purified carnosine (PC), leucyl-histidine (LH), homocarnosine (HC), histidine (His), and beta-alanine (beta-Ala) to scavenge peroxyl radicals generated by 2,2´-azobis-(2-amidopropane) (AAPH) as determined by the peroxyl radical induced fluorescence decay of beta-phycoerythrin (beta-PE): 1) beta-PE; 2) beta-PE +AAPH; 3-7) the same + beta-Ala (3), His (4), HC (5), PC (6), or LH (7). All peptides and amino acids were added at 0.25 mM.
Fig. 2. Ability of purified carnosine (10 mM) to inhibit the oxidation of phosphatidylcholine liposomes (2 mg/ml) in the presence of Fe3+ (15 µM) or Cu2+ (30 µM) and ascorbate (100 µM). Oxidation was determined by measuring thiobarbituric acid reactive substances and was expressed as percent inhibition in comparison to no carnosine controls: 1) pentane (copper + ascorbate); 2) hexanal (copper + ascorbate); 3) hexanal (iron + ascorbate); 4) pentane (iron + ascorbate).
Fig. 3. Ability of varying carnosine concentrations (1-25 mM) to inhibit the oxidation of phosphatidylcholine liposomes (2 mg/ml) in the presence of Fe3+ (15 µM) or Cu2+ (30 µM) and ascorbate (100 µM). Oxidation was determined by measuring headspace hexanal or pentane: 1) copper + ascorbate; 2) iron + ascorbate.
CONJUGATION OF ALDEHYDIC LIPID OXIDATION PRODUCTS BY PURIFIED CARNOSINE
The ability of biomolecules to interfere with or delay the formation of primary lipid oxidation products (e.g., lipid peroxides and free radicals) has been the focus of most antioxidant studies. However, biomolecules can also interact with potentially toxic secondary lipid oxidation products that arise from the decomposition of lipid peroxides. Therefore, the ability of carnosine to interact with aldehydic lipid oxidation products was re-evaluated using purified carnosine. Oxidation of lipids in biological tissues and foods results in the formation of a variety of aldehydic compounds, including saturated, alpha,beta-monounsaturated, polyunsaturated, and hydroxylated aldehydes [7, 8]. These aldehydic oxidation products will react with biomolecules causing various deleterious effects in foods (e.g., loss of protein functionality and discoloration of myoglobin) [9, 10] and in biological tissues (e.g., oxidative modification of DNA, enzymes, and lipoproteins) [11-15].
Purified carnosine will decrease the concentration of both saturated and unsaturated aldehydes commonly found in oxidized lipids. Interactions of carnosine with aldehydes are greatest for alpha,beta-monounsaturated (e.g., trans-2-hexenal and trans-2-nonenal) followed by polyunsaturated (e.g., trans,trans-2,4-hexadienal and trans,trans-2,4-decadienal) and saturated (e.g., hexanal and propanal) aldehydes [16, 17]. Kinetic studies showed that the reaction between carnosine and trans-2-hexenal is second-order overall, and first-order in each of the two reactants. This was supported by the following observations: 1) at 0.5 mM of trans-2-hexenal concentration and pH 7.4, increase in carnosine concentration was linearly proportional to the decrease in trans-2-hexenal concentrations in phosphate buffer [18]; 2) at fixed carnosine concentration of 5 mM and pH 5 to 8, straight lines were obtained when reaction time (t) was plotted against ln(a/ax), where a and ax represent concentrations of trans-2-hexenal at the beginning or during the course of reaction, respectively.
Carnosine was much more effective in quenching trans-2-hexenal than its constituent amino acids, histidine and beta-alanine. At pH 7.4, carnosine reacted with trans-2-hexenal 3.3 times faster than histidine (16.6·10-4 versus 3.0·10-4 mM-1·min-1). Little interaction was observed between beta-alanine and trans-2-hexenal under the same conditions [16]. Carnosine and histidine have similar hexanal quenching activity (both <5% reduction compared to 44 and 8% reduction of trans-2-hexenal by carnosine and histidine, respectively) [16, 17]. Structurally related dipeptides such as leucyl-histidine and isoleucyl-histidine decrease headspace hexanal concentrations over 3 times more effectively than carnosine [17]. Increase in pH from 5 to 8 substantially accelerated the reaction between trans-2-hexenal and carnosine, while only a slight increase was observed between trans-2-hexenal and histidine under the same conditions [18].
Several other compounds in biological tissues can also form conjugates with aldehydes, including glutathione, lipoic acid, and polyamines. On an equal molar basis, the ability of nucleophilic compounds common to skeletal muscle to decrease the concentration of the alpha,beta-unsaturated aldehyde trans-2-hexenal is in the order glutathione > carnosine > spermine > spermidine [16]. The ability of these same compounds to decrease the concentration of the saturated aldehyde hexanal is in the order glutathione > spermine > spermidine = carnosine [17]. However, the typical concentration of these compounds in skeletal muscle is in the order of carnosine (2800 mg/kg porcine muscle) > glutathione (69 mg/kg turkey muscle) > spermine (34 mg/kg porcine muscle) > spermidine (3 mg/kg porcine muscle) [16, 17]. Since carnosine concentrations are 40- to over 900-fold greater than these other nucleophiles, it is likely that carnosine would have the greatest potential for decreasing the concentration of toxic aldehydic lipid oxidation products in skeletal muscle. For example, when the ability of purified carnosine and glutathione to decrease the concentrations of trans-2-hexenal is compared at typical skeletal muscle concentrations (10 and 0.3 mM, respectively), carnosine is much more effective, decreasing trans-2-hexenal 67% compared to 19% for glutathione.
While many aldehydic lipid oxidation products are potentially toxic to biological tissues, the greatest attention has been focused on the toxicity of 4-hydroxy-2-trans-nonenal. Carnosine decreases the concentrations of 4-hydroxy-2-trans-nonenal in a manner similar to alpha,beta-unsaturated aldehydes. 4-Hydroxy-2-trans-nonenal (0.5 mM) concentrations were reduced over 80% by purified carnosine (5 mM) after 3 h of incubation at 40°C and pH 7.4 [16]. These data suggests that carnosine may be able to substantially reduce the toxicity of 4-hydroxy-2-trans-nonenal in skeletal muscle. However, more research is needed to determine how the rates of carnosine-4-hydroxy-2-trans-nonenal interactions compare to other biomolecules.
CHANGES IN CARNOSINE, ANSERINE, AND alpha-TOCOPHEROL
CONCENTRATIONS IN OXIDIZING SKELETAL MUSCLE
In order to better understand the importance of carnosine as a skeletal muscle antioxidant, changes in carnosine, anserine, and alpha-tocopherol concentrations were determined during the oxidation of turkey skeletal muscle. In both breast and thigh muscle, alpha-tocopherol oxidation rates were much greater than carnosine and anserine [18]. In fact, carnosine and anserine concentrations were not observed to substantially decrease until fatty acid oxidation had already begun. The fact that carnosine and anserine are oxidized at slower rates than alpha-tocopherol and fatty acids suggests that the ability of carnosine and anserine to scavenge free radicals may not be important in vivo. However, the ability of carnosine and anserine to chelate metals could play an important role in the overall oxidation rates of skeletal muscle, and loss of carnosine and anserine during the latter stages of oxidation could indicate that these dipeptides are forming conjugates with aldehydic lipid oxidation products.
Thus, carnosine is a multifunctional antioxidant that can inactivate free radicals, chelate prooxidative metals such as copper, and form conjugates with potentially toxic aldehydic lipid oxidation products. Further research is needed to determine if many of the initial reports of the antioxidant activity of carnosine are accurate due to the potential contamination of synthetic sources of carnosine by hydrazine. In addition, further research is needed to identify specific carnosine oxidation products and to develop methods to measure the presence of carnosine-aldehyde adducts in biological tissues. Without better clarification of the antioxidant activity of carnosine in vivo, we will not understand the antioxidant role of carnosine and its related dipeptides in biological tissues.
REFERENCES
1.Boldyrev, A. A., Dupin, A. M., Pindel, E. V., and
Severin, S. E. (1988) Comp. Biochem. Physiol., 89B,
245-250.
2.Kohen, R., Yamamoto, Y., Cundy, K. C., and Ames, B.
(1988) Proc. Natl. Acad. Sci. USA, 85, 3175-3179.
3.Decker, E. A., Crum, A. D., and Calvert, J. T.
(1992) J. Agric. Food Chem., 40, 756-759.
4.Aruoma, O. I., Laughton, M. J., and Halliwell, B.
(1989) Biochem. J., 264, 863-869.
5.Zhou, S., Dickinson, C., Yang, L., and Decker, E.
A. (1998) Analyt. Biochem., 261, 79-86.
6.Gantt, E., and Lipschultz, C. A. (1974)
Biochem., 13, 2960-2966.
7.Poli, G., Dianzani, M. U., Cheeseman, K. H.,
Slater, T. F., Lang, J., and Esterbauer, H. (1985) Biochem. J.,
227, 629-638.
8.St. Angelo, A. J., Vercellotti, J. R., Legendre, M.
G., Vinnett, C. H., Kuan, J. W., James, C., and Dupuy, H. P. (1987)
J. Food Sci., 52, 1163-1168.
9.Xiong, Y. L., and Decker, E. A. (1995) J. Muscle
Foods, 6, 139-160.
10.Chan, W. K. M., Faustman, C., and Decker, E. A.
(1997) J. Food Sci., 62, 709-712.
11.Brooks, B. R., and Klamerth, O. L. (1968) Eur.
J. Biochem., 5, 178-182.
12.Reiss, U., Tappel, A. L., and Chio, K. S. (1972)
Biochem. Biophys. Res. Commun., 48, 921-926.
13.Yau, T. M. (1979) Mech. Ageing Develop.,
11, 137-144.
14.Szweda, L. I., Uchida, K., Tsai, L., and
Stadtman, E. R. (1993) J. Biol. Chem., 268,
3342-3347.
15.Alaiz, M., Beppu, M., Ohishi, K., and Kikugawa,
K. (1994) Biol. Pharm. Bull., 17, 51-57.
16.Zhou, S., and Decker, E. A. (1999) J. Agric.
Food Chem., 47,51-55.
17.Zhou, S., and Decker, E. A. (1999) J. Agric.
Food Chem., 47, 1932-1935.
18.Decker, E. A., Livisay, S. A., and Zhou, S.
(2000) in Antioxidants in Muscle Foods (Faustman, C., and
Decker, E. A., eds.) John Wiley and Sons, N. Y., in press.