Autotrophic Assimilation of CO2 and C1-Compounds by Pathogenic Bacteria
L. S. Buzolyova* and G. P. Somov
Institute of Epidemiology and Microbiology, Siberian Branch of the Russian Academy of Medical Sciences, ul. Sel'skaya 1, Vladivostok, 690028 Russia; fax: (4232) 294-303* To whom correspondence should be addressed.
Received February 5, 1999; Revision received April 28, 1999
We demonstrate for the first time that the pathogenic bacteria Yersinia pseudotuberculosis and Listeria monocytogenes (pathogens of saprozoonoses) are capable of chemolithoautotrophic assimilation of CO2. Low temperature is favorable for better absorption of CO2 by these bacteria; this is supported by increased enzymatic activity of carbonic anhydrase acting as the supplier of the substrate to the site of carboxylation. Data of radioisotopic methods indicate that assimilated labeled carbon of CO2 is incorporated into all major cell biopolymers. The bacteria can utilize not only CO2, but also other C1-compounds for biosynthesis.
KEY WORDS: saprozoonoses, autotrophy, Yersinia pseudotuberculosis, Listeria monocytogenes, carbonic anhydrase, C1-compounds
The existence of a saprophytic phase in the life cycle of some bacterial pathogens is now clear [1, 2]. For these microorganisms, the environment is of high ecological and epidemiological importance, including soil, water, and plant substrates where they can grow and persist [3-5]. The main requirement for the saprophyte phase of the pathogens is a wide ecological tolerance, i.e., preservation of viability of the population during large changes in temperature, humidity, levels of organic and inorganic compounds, etc. Unlike the parasitic phase inside a human or animal host, when the pathogens of saprozoonoses receive organic nutrients metabolized heterotrophically, the saprophytic phase of the bacterial life cycle in the environment includes limited nutrition and unstable temperature. However, even under severe conditions, the pathogenic bacteria can survive and grow. Pathogens of pseudotuberculosis and listeriosis were isolated from soil and water during the cold seasons [3, 6]. The bacteria experimentally inoculated in tap or distilled water survive for a long time (up to one year) and under certain conditions, they even grow [7]. The studies suggest amazing viability of the pathogens of saprozoonoses, confirming their possible saprophyte growth. On the other hand, the mechanisms providing for growth of pathogenic bacteria at low temperature under poor trophic conditions are unknown.
Many saprophytic bacteria have extensive adaptivity determined by genetic and biochemical mechanisms which allow them to survive in constantly changing environments. Saprophytic bacteria quantitatively dominate in natural ecosystems and can grow at very low concentrations of organic nutrients and low temperature; many of these bacteria are capable of autotrophic assimilation of carbon [8, 9]. Thus, similar to saprophytic bacteria, pathogenic microorganisms living in soil and water can be also autotrophic.
The goal of the present study was to investigate the possible autotrophic metabolism in certain pathogenic bacteria (pathogens of saprozoonoses), Yersinia pseudotuberculosis and Listeria monocytogenes.
MATERIALS AND METHODS
Strains of Yersinia pseudotuberculosis Nos. 512, 907, 2781, and 282 were from the Museum of the Russian Center of Yersinioses (Institute of Epidemiology and Microbiology, Siberian Branch of the Russian Academy of Medical Sciences, Vladivostok) and strains of Listeria monocytogenes P, K, A, and 10CN were from the Russian State Control Institute of Veterinary Drugs (Moscow); all strains had typical morphological, culture, biochemical, and antigenic characteristics.
Bacteria grown in slant agar were washed three times with physiological saline and plated in 300 ml of Hirsch medium containing (g/liter): KH2PO4, 1.36; Na2HPO4, 3.3; (NH4)2SO4, 0.5; MnSO4, 0.25; Na2MoO4, 0.25; MgSO4, 0.2; CuCl2, 0.01; FeSO4, 0.005 [10]. The initial concentration was 1000 bacterial cells/ml of the medium according to the optical density standard. Number of colony-forming bacterial cells in 0.1 ml of the medium (colony-forming units; CFU/0.1 ml) was determined by plating on broth--peptone agar (BPA) and expressed as the logarithm of the number.
Assimilation of gases by the bacteria was studied at 6-8 and 20-25°C in liquid Hirsch medium without carbon sources in glass vials sealed air-tight with rubber stoppers fixed by metal caps. Carbon dioxide (3%) or hydrogen (10%) were injected by syringe through the rubber stopper into the vials. Higher fractions of the gases inhibits hydrogenase activity of the cultures [11].
Changes in hydrogen and carbon dioxide concentration in the gaseous phase were registered using a LKhM-8MD chromatograph (model 3700). A SOVPOL column (2.5 m × 3 mm) was used for carbon dioxide analysis: carrier gas, argon; column temperature, 70°C; detector temperature, 80°C; gas pressure, 1.4 atm. To analyze the hydrogen content, a molecular sieve 5A column (2 m × 3 mm) was used: carrier gas, argon; column temperature, 130°C; detector temperature, 120°C; gas pressure, 1 atm [12].
Gas assimilation by bacteria grown in liquid medium was monitored in parallel with determination of the growth kinetics of the cultures by calculating the number of colonies on a dense nutrient medium. The culture (0.1 ml) was aseptically removed by a syringe through the rubber stopper and plated in BPA at the necessary dilution.
Carbonic anhydrase activity was determined in crude extracts prepared by sonication of bacterial cells. The bacteria grown at 6-8 and 20-25°C were separated from the culture medium (broth--peptone agar) and washed twice with physiological saline and three times with 0.025 M veronal buffer (pH 8.2). The cells were suspended in the latter buffer and disrupted by ultrasound (UZDN-1 apparatus; 3 cycles for 2 min at 44 kHz). The suspension was centrifuged at 7000 rpm for 30 min. To remove low-molecular-weight compounds interfering with enzyme activity assay, the supernatant was dialyzed against 0.025 M veronal buffer (pH 8.2).
Bacterial carbonic anhydrase was assayed by the method of Romanova and Elinova [13] using bromothymol blue indicator or a pH meter. When the indicator was used, the time of transformation of blue color into yellow was registered. Control assays were performed in the presence of boiled enzyme. Carbonic anhydrase activity was expressed in arbitrary units (according to Wilbur and Anderson) calculated as described [13]. Protein was assayed by the Lowry method.
When the pH meter was used to assay the activity of carbonic anhydrase, the rate of acidification was measured. The reaction was performed in a glass flask in an ice-water bath. The control assay included 6 ml of 0.025 M veronal buffer (pH 8.2). Four milliliters of saturated (0°C) CO2 solution were carefully removed (to avoid possible gas leakage) and transferred into the flask with the buffer. The initial rate of pH decrease was determined. Experimental samples included 5.9 ml of the buffer and 0.1 ml of enzyme preparation (protein concentration 2.8 ± 0.2 mg/ml).
CO2 assimilation and CO2-dependent synthesis of cellular biopolymers were confirmed by the radioisotope method with 14CO2. Experiments were performed in a gas chamber containing 10% CO2, and labeled sodium bicarbonate (8.5 µCi) was added directly to the mineral Hirsch medium (pH 7.2-7.4) [13]. Bacteria (1000 cells/ml medium) were temporarily cultured at 6-8 and 20-25°C. Assimilation activity of the microbial strains was studied in the exponential growth phase. The bacteria were pelleted by centrifugation (7000-10000 rpm), and the culture medium was washed away twice with physiological saline; then, 14C content was determined in the last portion of the supernatant. The precipitate was fixed with 10% trichloroacetic acid (TCA). Total assimilated radioactivity was determined in a aliquot of the suspension. Soluble assimilation products were extracted by sequential treatment of the bacterial suspension in the cold with 5 and 10% TCA. The amount of biopolymers synthesized by the bacterial cells was determined after fractionation of low- and high-molecular-weight compounds by the methods recommended by Kennel [14]. Protein content was assayed by the Lowry method after 15 min hydrolysis of the cell suspension in 0.5 M NaOH at 105°C, and carbohydrates were assayed by the anthrone method [15]; nucleic acids were determined by the method of Schmidt--Tannhauser as modified by Korotyaev and Krolichenko [16], and lipids were determined by the method of Blur as modified by Bragdon [17]. Before the assay, lipids were purified from non-lipid contaminations [18]. The activity of labeled compounds was determined using a Rack-Beta II scintillation counter (LKB, Sweden), and statistical significance of the data was calculated (I95) [19].
Consumption of C1-compounds was studied in the liquid mineral Hirsch medium and distilled water containing methanol, sodium formate, and dimethylamine at concentrations 0.5, 1, 1.5, and 3%. Cultures were grown at 37, 20-25, and 6-8°C. Methanol content in the medium was assayed by the bichromate method based on the development of color reaction during methanol oxidation with potassium bichromate [11]. Cells were pelleted and 5 ml of 0.4 N K2Cr2O7 solution was added to 1 ml of culture medium; the volume of the mixture was adjusted to 25 ml in a volumetric flask with distilled water, and the optical density of the solution was determined at 590 nm (SF-47 spectrophotometer). Assays were repeated in 6-9 parallel samples. Statistical evaluation of the data was performed using an IBM-PC and Excel spreadsheet software version 7. The significance of the differences between the groups was evaluated by Student's t-test.
RESULTS AND DISCUSSION
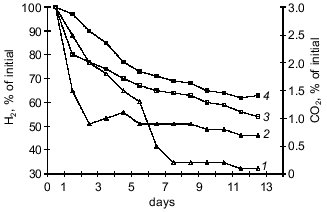
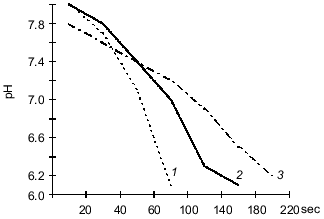
The data indicate that the investigated bacterial species can utilized CO2 from a gas--air mixture. The experiments with Y. pseudotuberculosis (Fig. 1) performed at 20-25°C indicate that consumption of CO2 (that was the only carbon nutrient) from the gas phase is more intense during the first 24 h of bacterial growth. At 6-8°C, consumption of CO2 by the cells was active on days 6-7 of growth corresponding to the exponential growth of the "cold" variant. The amount of carbon dioxide consumed at the beginning of the steady-state growth phase was also higher in the "cold" variant (95 ± 1.4%) versus the "warm" variant (76 ± 1.6%) of bacterial growth (p < 0.01). This can be explained by the activity of carbonic anhydrase which supplies the substrate to the carboxylation site [13]. To check this hypothesis, in experiments with L. monocytogenes it was shown that during the steady-state growth phase, the activity of carbonic anhydrase determined as the time of indicator color transition is 4-fold higher in the "cold" variant (10.8 ± 1.21) than the "warm" variant (2.48 ± 0.34) (p < 0.01). The data were confirmed by determination of carbonic anhydrase activity as the rate of medium acidification (Fig. 2).
Fig. 1. Changes in carbon dioxide content in gas--air mixture during the oxidation of hydrogen by Y. pseudotuberculosis (strain 512) in Hirsch medium (initial concentration of bacteria was 1000 cells/ml): 1) consumption of carbon dioxide at 20-25°C; 2) consumption of carbon dioxide at 6-8°C; 3) oxidation of hydrogen at 6-8°C; 4) oxidation of hydrogen at 20-25°C.
Assimilation of CO2 is responsible for the main consumption of high-energy compounds and is tightly associated with cell energetics. A correlation between CO2 consumption and increased hydrogenase activity of the cultures suggests that intense oxidation of hydrogen is accompanied by more intense assimilation of CO2 due to autotrophic carbon fixation by the Y. pseudotuberculosis and L. monocytogenes cells. It should be noted that the energy demand in the "cold" culture variants during autotrophic growth is higher than that of the "warm" variants because at 6-8°C the bacteria consumed more hydrogen than at 20-25°C.Fig. 2. Carbonic anhydrase activity in L. monocytogenes (strain A) grown on BPA (broth--peptone agar) at 6-8°C (1) and 20-25°C (2) (protein concentration, 2.8 ± 0.2 mg/ml); 3) control (without the enzyme).
Possible autotrophic assimilation of inorganic carbon and synthesis of the main cellular biopolymers from that carbon was confirmed by the radioisotopic method with 14CO2. The strain 2781-H of the pseudotuberculosis pathogen isolated from a patient and a soil variant of this strain (the strain was incubated in a soil model ecosystem for nine months during several seasons of the year) were used. The data indicate that this strain of the pseudotuberculosis pathogen assimilates 14CO2 24 h after contact with the labeled material. At 6-8°C, 31.2 ± 4.6% of the 14C was utilized, and at 20-25°C, 20.1 ± 2.3% was utilized. The soil variant of the strain assimilated 14C more actively. At 6-8°C, 77.2 ± 2.6% was assimilated and at 20-25°C, 45.7 ± 2.2% was assimilated. Labeled carbon was incorporated in all major biopolymers of the cell, including proteins (70 ± 4.2%), carbohydrates (4.4 ± 0.4%), lipids (10 ± 1.0%), RNA (2.8 ± 0.6%), and DNA (2.8 ± 0.9%). Assimilated carbon was predominantly directed to biosynthesis of products with highly reduced organic acids (amino acids), thus indirectly suggesting that carbon dioxide is assimilated by the reducing pentose phosphate cycle of Calvin and Benson [20]. Hence, the pathogenic bacteria can be considered as facultative autotrophs which can use carbon dioxide as the sole carbon source with energy derived by the oxidation of hydrogen under certain conditions.
A type of nutrition based on oxidation of C1-compounds is a variant of chemolithotrophy; this is highly specialized energy-providing pathway combined with typical autotrophic carbon metabolism [8, 9]. Thus, the chemolithoautotrophic hypothesis is consistent with studies on the ability of bacteria to biosynthetically utilize carbon originating not only from carbon dioxide, but from other single-carbon-atom-containing compounds as well.
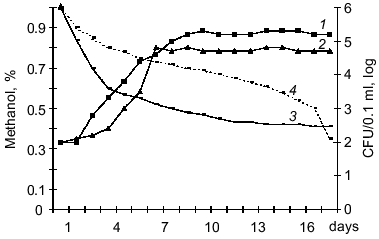
The L. monocytogenes and Y. pseudotuberculosis strains can grow on methanol, formate, and dimethylamine. Methanol was most preferential substrate for bacterial growth, and dimethylamine the least preferential substrate. The growth rate on C1-compounds varied between the strains. Listeria strains can be arranged in the following order corresponding to decreasing growth rate on these substrates (maximal concentration CFU/0.1 ml): A (5 ± 0.5 log), 10CN (5 ± 0.2 log), P (4 ± 0.8 log), and K (3 ± 0.7 log). Yersinia strains can be arranged in the order: 512 (5 ± 0.8 log), 2781-H (5 ± 0.5 log), 907 (5 ± 0.3 log), and 282 (2 ± 0.1 log). Optimal growth was observed at concentrations of C1-compounds from 0.1 to 1% and higher concentrations (2-3%) suppressed the growth of Listeria and Yersinia. The highest rate of methanol consumption was detected in strains 512 and 2781-H of Y. pseudotuberculosis (Fig. 3) and in strain A of L. monocytogenes. However, the specific growth rate of Y. pseudotuberculosis on methanol exceeded that of Listeria by 1.5-2-fold. The optimal temperature for growth of the bacteria on C1-compounds was 20-25°C, whereas at 6-8°C specific growth rate of bacterial in log phase was 2-3-fold lower, and at 37°C the bacterial cells died. Similar to obligatory autotrophs, the carbon of C1-compounds may be assimilated autotrophically, i.e., after oxidation of CO2 in the ribulose diphosphate cycle although it is possible that a fraction of methanolic carbon can be assimilated at a more reduced level (formaldehyde, formate) as methyl derivatives of tetrahydrofolic acid [21, 22]. However, this issue requires further studies including the assay of activities of key enzymes.
Thus, this work is the first to demonstrates that pathogenic bacteria which are the pathogens of saprozoonoses can autotrophically fix carbon in the environment via a chemolithoautotrophic metabolic pathway. Numerous saprophytic bacteria from various taxonomic groups can grow autotrophically, and detection of a similar property in the pathogenic bacteria L. monocytogenes and Y. pseudotuberculosis is of special importance. Pathogens of saprozoonoses are widespread in nature and depending on ecological niche, autotrophic metabolism of these bacteria is combined with possibilities of heterotrophic and mixotrophic growth. This provides their great advantage in preserving viability under unfavorable environmental conditions and can be considered as a reservoir of the pathogens.Fig. 3. Growth of Y. pseudotuberculosis (strain H-2781) on Hirsch medium containing 0.1% methanol at 20-25°C (1) and 6-8°C (2) and methanol consumption at 20-25°C (3) and 6-8°C (4). Initial concentration of bacteria was 1000 cells/ml medium.
This work was supported by the Russian Foundation for Basic Research (grant No. 97-4-48534).
REFERENCES
1.Somov, G. P. (1985) Zh. Mikrobiol. Epidemiol.
Immunol., 5, 98-104.
2.Litvin, V. Yu. (1985) Zh. Mikrobiol. Epidemiol.
Immunol., 6, 98-103.
3.Gershun, V. I. (1981) Issues on Natural Hotbeds
of Diseases [in Russian], Kainar, Alma-Ata.
4.Somov, G. P., and Litvin, V. Yu. (1988)
Saprophitism and Parasitism of Pathogenic Bacteria (Ecological
Aspects) [in Russian], Nauka, Novosibirsk.
5.Pokrovsky, S. V. (ed.) (1986) Handbook on
Infectious Diseases [in Russian], Meditsina, Moscow, p. 240.
6.Kuznetsov, V. G. (1983) Zh. Gigiena
Sanitariya, 2, 72-74.
7.Rozhkova, L. P. (1977) Epidemiology of
Far-Eastern Scarlet-Like Fever (Human Pseudotuberculosis): Author's
abstract of dissertation, Moscow.
8.Gutina, V. I. (1988) Issues on History of
Physiology of Microorganisms [in Russian], Nauka, Moscow.
9.Zavarzin, G. A. (1972) Litotrophic
Microorganisms [in Russian], Nauka, Moscow.
10.Hirsch, P., and Conti, T. (1964) J. Arch.
Microbiol., 48, 339.
11.Slabova, O. I., and Nikitin, D. I. (1988)
Mikrobiologiya, 57, 373-377.
12.Jeffery, P., and Kippish, P. (1976) Analysis
of Gases by Gas Chromatography Methods [Russian translation], Mir,
Moscow.
13.Romanova, A. K. (1980) Biochemical Methods of
Study of Autotrophy in Microorganisms [in Russian], Nauka,
Moscow.
14.Kennel, D. (1967) Meth. Emzymol.,
12A, 686-692.
15.Gerhardt et al. (ed.) (1984) Methods of
General Bacteriology [Russian translation], Vol. 2, Mir, Moscow, p.
293.
16.Korotyaev, A. I., and Krolichenko, T. P. (1976)
Zh. Mikrobiol. Epidemiol. Immunol., 11, 64.
17.Pokrovsky, A. A. (1963) Biochemical Methods of
Clinical Studies [in Russian], Medgiz, Moscow.
18.Cates, M. (1975) Techniques in Lipidology
[Russian translation], Mir, Moscow.
19.Dois, E. A. (1983) Quantitative Problems of
Biochemistry [Russian translation], Mir, Moscow.
20.Romanova, A. K., and Elinova, E. E. (1980)
Usp. Mikrobiol., 15, 23-40.
21.Loginova, N. V., Govorukhina, N. I., and
Trotsenko, Yu. A. (1981) Mikrobiologiya, 50, 591.
22.Cox, R. B., and Quagle, S. R. (1975) Biochem.
J., 150, 569.