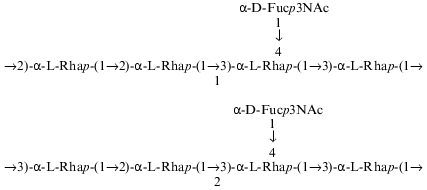Structure of the O-Polysaccharide of the Lipopolysaccharide of Pseudomonas syringae pv. garcae ICMP 8047
E. L. Zdorovenko1, V. V. Ovod2, A. S. Shashkov1, N. A. Kocharova1, Yu. A. Knirel1*, and K. Krohn2
1Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninskii pr. 47, Moscow, 117913 Russia; fax: (095) 135-5328; E-mail: knirel@ioc.ac.ru2Institute of Medical Technology, University of Tampere, P.O. Box 607, Tampere, 33101 Finland; fax: (358-3) 215-7332; E-mail: ltvlov@uta.fi
* To whom correspondence should be addressed.
Received January 11, 1999
The composition and structure of the O-polysaccharide of the lipopolysaccharide of Pseudomonas syringae pathovar garcae ICMP 8047 were studied using methylation analyses, Smith degradation, and 1H- and 13C-NMR spectroscopy, including two-dimensional correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY), nuclear Overhauser effect spectroscopy (NOESY), and H-detected 1H,13C heteronuclear multiple-quantum coherence (HMQC) experiments. The polysaccharide was found to contain L-rhamnose and 3-acetamido-3,6-dideoxy-D-galactose (D-Fuc3NAc) in the ratio 4:1 and to consist of two types of pentasaccharide repeating units. The major (1) and minor (2) repeating units differ from each other only in the position of substitution of one of the rhamnose residues in the main chain. Similar structural heterogeneity has been reported formerly in O-polysaccharides of some other P. syringae strains having a similar monosaccharide composition. A Fuc3NAc residue is attached to the main rhamnan chain as a side chain by a (alpha1-->4) glycosidic linkage; this has not hitherto been described in P. syringae:
KEY WORDS: lipopolysaccharide, O-specific polysaccharide, 3-acetamido-3,6-dideoxy-D-galactose, structural heterogeneity, Pseudomonas syringae