Immunochemical Characterization of Steroid Hydroxylases of Adrenocortical Mitochondria. 1. Antibodies as Inhibitors of Cytochrome P450scc (CYP11A1) and P45011beta (CYP11B1) Activities
A. A. Chernogolov1,2 and S. A. Usanov1
1Institute of Bioorganic Chemistry, Academy of Sciences of Belarus, ul. Zhodinskaya 5/2, Minsk, 220141 Belarus; fax: (375-17) 263-7274; E-mail: usanov@ns.iboch.ac.by2To whom correspondence should be addressed.
Submitted July 4, 1997.
The effects of antibodies against protein components of the monooxygenase systems of adrenocortical mitochondria on the reactions of hydroxylation of cholesterol and 11beta-deoxycorticosterone were investigated in a reconstituted system containing cytochromes P450scc (CYP11A1) and P45011beta (CYP11B1) as the terminal oxidases and the electron-transfer proteins adrenodoxin reductase and adrenodoxin. It has been shown that affinity-purified antibodies to cytochromes P450scc and P45011beta are efficient modulators of the activity of these systems, and their inhibiting effect is mainly due to interference with the interaction of heme proteins and adrenodoxin. The antibodies against polypeptide fragments of the cytochrome P450scc molecule F1 (Ile1-Arg256), F2 (Asn257-Ala481), and F3 (Asn257-Arg399) were used to demonstrate that the interaction of heme protein with adrenodoxin has a multisite character and involves regions located in the N- and C-terminal sequences of cytochrome P450scc.
KEY WORDS: adrenal cortex, cytochrome P450scc, cytochrome P45011beta, adrenodoxin, adrenodoxin reductase, antibodies, steroids.
Abbreviations: AR) adrenodoxin reductase; AD) adrenodoxin; P450scc) cytochrome P450 catalyzing cholesterol side-chain cleavage reaction; P45011beta) cytochrome P45011beta.
The syntheses of mineral- and glucocorticoids in adrenocortical
mitochondria involve cytochrome P450-dependent monooxygenase systems
consisting of adrenodoxin reductase (AR)3, adrenodoxin (AD),
and cytochromes P450scc and P45011beta [1, 2]. Investigation of the
mechanism of the monooxygenase reactions for the mitochondrial
cytochromes P450, responsible for the key reactions of cholesterol
hydroxylation and steroid 11beta-hydroxylation in adrenal cortex
and other steroidogenic tissues, is of principal importance.
Antibodies against various components of the monooxygenase systems are widely used to elucidate the role of cytochrome P450 in metabolism of endogenous [3-7] and exogenous [8-13] compounds. Usually, one of the following effects is observed: the antibodies either fail to affect any step of the monooxygenase cycle, or they inhibit one of them. Of the most interest are antibodies affecting the activity of the terminal monooxygenase components, including those against functionally important polypeptide fragments of cytochrome P450. These antibodies represent a potent tool for structural--functional studies of cytochrome P450 and investigation of the mechanism of P450-catalyzed reactions.
Antibodies are widely used in investigating the specificity of protein--protein interactions in microsomal cytochrome P450-dependent systems [14, 15]. As compared to microsomal systems, the mitochondrial monooxygenases have a more complex electron transfer system consisting of AR, AD, and cytochrome P450scc or P45011beta. As shown previously, addition of antiserum to cytochrome P450scc in a reconstituted system containing AR, AD, and cytochrome P450scc inhibits the conversion of cholesterol to pregnenolone [16-19]. The antibodies against large fragments of cytochrome P450scc molecule F1 and F2, corresponding to its N- and C-terminal sequences, also inhibit this reaction [19, 20].
The goal of the present study was to investigate the effect of affinity-purified antibodies against the components of the cholesterol- and 11beta-hydroxylation systems on hydroxylations of cholesterol and 11beta-deoxycorticosterone in the reconstituted system containing NADPH, AR, AD, and the corresponding heme protein at various stoichiometric ratios. To investigate the mechanism of inhibitory action of antibodies and to determine the functional role of structural elements of the heme protein, antibodies against polypeptide fragments of the cytochrome P450scc molecule were used. It has been shown that affinity-purified antibodies against cytochromes P450scc and P45011beta efficiently inhibit enzymatic activity of the indicated systems, mainly by interfering with cytochrome P450scc--AD interaction. Based on the data obtained, we conclude that the cytochrome P450scc--AD interaction involves regions located in N- and C-terminal sequences of the heme protein molecule and is referred to as a multisite type of interaction.
MATERIALS AND METHODS
Chemicals. Chemicals used in the present study were the following: NADPH, glucose-6-phosphate, and glucose-6-phosphate dehydrogenase from Boehringer (Germany); cholesterol, pregnenolone, 11beta-deoxycorticosterone, trypsin from bovine pancreas (45 units per mg), soybean trypsin inhibitor, EDTA, sodium dithionite, Tris, sodium cholate, Tween 80, and silica gel plates Silufol UV-254 from Serva (Germany); [1alpha,2alpha(n)-3H]cholesterol (58 Ci/mmole) from Amersham (UK); complete Freund’s adjuvant from Calbiochem (USA); Tween 20 from Merck (Germany); CNBr-activated Sepharose 4B and Sephadex G-25 (medium) from Pharmacia (Sweden).
Isolation and Characterization of Proteins. AR, AD, cytochromes P450scc and P45011beta were isolated from bovine adrenal cortex as described previously [21, 22]. The proteins were homogeneous by electrophoresis and immunochemical analysis and were characterized by the following spectrophotometric indices: A414/A280 = 0.85-0.87 for AD, A450/A272 = 7.2-7.7 for AR, A393/A280 = 0.85-0.88 for cytochrome P450scc, and A393/A280 = 0.9-1.1 for cytochrome P45011beta. Concentrations of AR and AD were determined spectrophotometrically using molar absorption coefficients 9.8 mM-1·cm-1 at 414 nm and 11 mM-1·cm-1 at 450 nm, respectively. Concentrations of cytochromes P450scc and P45011beta were determined from the difference spectra of the reduced heme protein and its carbon monoxide complex using molar absorption coefficient 91 mM-1·cm-1 at 450 nm [23].
Preparation of Polypeptide Fragments of Cytochrome P450scc. Limited proteolysis of the heme protein by trypsin at 25°C in 50 mM sodium phosphate buffer (pH 7.4) for 30 min at enzyme/substrate ratio 1:50 to prepare fragments F1 and F2 or for 60 min at the ratio 1:20 to prepare fragment F3 was performed. The fragments were separated and purified by thiol-disulfide exchange chromatography followed by preparative electrophoresis [19].
Preparation of Antisera and Isolation of Antibodies. Rabbit antisera to AR, AD, cytochrome P450scc, and fragments F1 and F2 were obtained as described previously [19, 20]. Antibodies against fragment F3 were raised by immunization of rabbits with lyophilized antigen preparation (150-200 µg) in polyacrylamide gel (prior to mixing with adjuvant, a buffer suspension of macerated gel was prepared). Cytochrome P450scc fragments were administered weekly for 9 weeks, the first three injections of fragments F1 and F2 and the first four injections of fragment F3 were intracutaneous, and the subsequent ones were subcutaneous. Immunization of rabbits with cytochrome P45011beta took 8 weeks with weekly subcutaneous injections of 150 µg of the antigen.
Total IgG fraction was isolated by fractionation of antisera with (NH4)2SO4, following with chromatography on DEAE-cellulose in 10 mM sodium phosphate buffer, pH 8.0 [24]. Affinity-purified IgG were isolated from preparations from ion-exchange chromatography, using columns with antigens immobilized on CNBr-activated Sepharose 4B according to the instructions of the manufacturer (Pharmacia). Affinity adsorbents contained (per ml of swollen gel): 5.6 mg cytochrome P450scc, 1 mg cytochrome P45011beta, 7.2 mg AD, 3 mg AR, 1.7 mg fragments F1 and F2 each, and 1.5 mg fragment F3. After IgG fraction was applied, adsorbents were subsequently washed with 50 mM sodium phosphate buffer (pH 7.4), then with 50 mM sodium phosphate buffer containing 1 M NaCl, 1 mM EDTA, and 0.3% sodium cholate (pH 7.4), and once more with 50 mM sodium phosphate buffer (pH 7.4). IgG fraction was eluted with 0.1 M glycine-HCl, pH 2.3, dialyzed against 5 mM sodium phosphate buffer containing 0.2 M NaCl (pH 7.0), and stored at -70°C.
Enzymatic Reduction of Cytochrome P450scc. Cytochrome P450scc reduction was measured at 20°C spectrophotometrically by formation of carbon monoxide complex of the reduced form of the heme protein [23]. Experimental and control cells each contained 1.2 ml 50 mM sodium phosphate buffer (pH 7.2) with 1 nmole cytochrome P450scc, 0.03 nmole AR, and 0.03 nmole AD. After the experimental cell was saturated with CO for 1 min, the reduction was started with addition of NADPH-regenerating system to both cells (final concentrations were 0.1 mM NADPH, 2 mM glucose-6-phosphate, and 1 unit per ml for glucose-6-phosphate dehydrogenase). The reaction rate was calculated by the increase in optical density at 450 nm in difference absorption spectrum.
Interaction of Cytochrome P450scc with Cholesterol and AD in the Presence of Antibodies. The effect of antibodies on cytochrome P450scc interaction with cholesterol and AD was examined by spectrophotometric titration [25]. The titration was carried out in 50 mM sodium phosphate buffer, pH 7.4, containing 0.05% Tween 20. The concentrations of cytochrome P450scc were 1 µM for titration with cholesterol and 2.9 µM for titration with AD. A solution of the protein was preincubated with antibodies and divided in two equal volumes added to cells with 0.5- and 1-cm optical path, for titrations with cholesterol and AD, respectively. After the baseline was recorded, a solution of effector was added to the experimental cell, and an equal volume of buffer was added in the reference cell. For titration with AD, one more couple of cells was used to compensate for its own absorption. Effectors were added in portions, and each time when equilibrium was reached (5-10 min), the difference spectra were recorded.
Enzymatic Activity of Cytochrome P450scc. Cytochrome P450scc enzymatic activity was determined in a reconstituted system as described previously [19]. The reconstituted system contained (unless specified otherwise) 1 µM cytochrome P450scc, 5 µM AD, 1 µM AR in 50 mM phosphate buffer containing 0.025% Tween 20, 50 µM cholesterol, and [3H]cholesterol (100,000 counts/min). After a 20-min preincubation, the reaction was started with addition of NADPH-regenerating mixture containing 0.1 mM NADPH, 1 mM glucose-6-phosphate, and glucose-6-phosphate dehydrogenase (1 unit per ml) (final concentrations). When examining the effects of antibodies against cytochrome P450scc and its fragments, antibodies (0.5-15 mg) were preincubated with the components of the reaction medium for 10-30 min, and then the reaction was started by addition of the NADPH-regenerating mixture.
The reaction was carried out in 1 ml final volume at 20°C for 1-5 min and stopped by addition of HCl to final concentration 0.1 M. The reaction mixture was twice extracted with 2 ml of ethyl acetate, and the extract was evaporated in a rotatory evaporator. The solid residue was dissolved in 10-20 µl ethyl acetate and applied to Silufol UV-254 plates. Cholesterol and pregnenolone were separated in the system hexane--ether--acetic acid (15:15:1 v/v), with a mixture of cholesterol and pregnenolone standards as the control. Chromatograms were developed in iodine vapor, and the stained regions corresponding to cholesterol and pregnenolone were cut out, put into vials with 6 ml ZhS-7 scintillation fluid, and counted for radioactivity on a Mark III counter (Tracor, France).
Enzymatic Activity of Cytochrome P45011beta. The activity of cytochrome P45011beta was determined in a reconstituted system (final volume 1 ml) containing 0.5 µM cytochrome P45011beta, 4 µM AD, 0.2 µM AR, and 100 µM 11beta-deoxycorticosterone. Proteins and the reaction substrate were mixed in 50 mM phosphate buffer containing 0.015% Tween 80 (pH 7.4), and the reaction was started by addition of the NADPH-regenerating mixture to the above-indicated final concentrations of components. The mixture was incubated for 2 min at 33°C and stopped by addition of 0.2 ml 0.5 M HCl. Reaction products were separated by HPLC on an Ultrasphere-ODS carrier in 0-80% acetonitrile gradient prepared in 0.1% CF3COOH on an Altex chromatograph (USA) with the detector set at 254 nm. Quantitative analysis of peaks was performed on a Chromatopak C-R1B (Shimadzu, Japan).
RESULTS
Effects of Anti-AR and Anti-AD Antibodies on Activities of Cytochromes P450scc and P45011beta. To determine the efficiency and specificity of antibodies raised against the protein components of mitochondrial cytochrome P450-dependent monooxygenases, the hydroxylating activities of cytochromes P450scc and P45011beta were studied in reconstituted systems in the presence of affinity-purified antibodies. Anti-AR and anti-AD antibodies inhibit both cytochrome P450scc- and cytochrome P45011beta-dependent reactions of hydroxylation (Table 1). For the ratio AR/AD/P450scc 1:5:1 and cholesterol concentration 50 µM, the amount of pregnenolone formed in 2 min in the reconstituted system decreased by 40% in the presence of 5-fold molar excess (with respect to AD) of the affinity-purified anti-AD antibodies. For the same protein ratio and 11beta-deoxycorticosterone concentration 100 µM in the presence of the same amount of anti-AD antibodies the activity of cytochrome P45011beta decreases by 35%. Therefore, independently of the terminal oxidase, anti-Ad antibodies inhibit the activities of both systems to a similar degree. Under the same conditions but in the presence of 10-fold molar excess of anti-AR antibodies, 14.1% of added cholesterol and 34.7% of 11beta-deoxycorticosterone are hydrolyzed (versus 22.6 and 40.2%, respectively, in the presence of non-immune antibodies).
TABLE 1. Enzymatic Activities of
Cytochromes P450scc and P45011beta in the Presence of
Antibodies
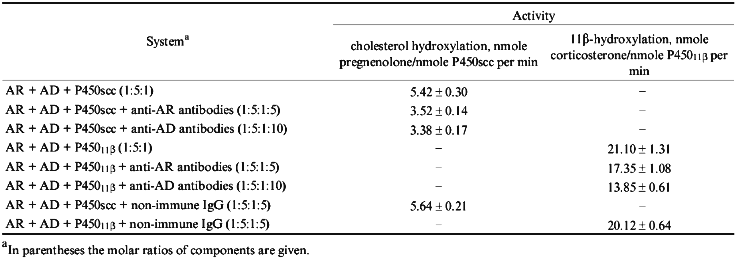
Thus, affinity-purified anti-AD antibodies inhibit the reactions catalyzed by cytochromes P450scc or P45011beta to a similar degree which does not exceed 40%, while for anti-AR antibodies the inhibiting effect is stronger and more specific in the cholesterol-hydroxylating system. What could be the reason for a relatively low inhibition of steroid conversion reactions by the antibodies against the intermediate protein carriers of the electron-transfer chain? Probably, the mechanism through which the antibodies inhibit the reactions of steroid oxidation is a complex one: antibodies may interfere with formation and/or dissociation of various intermediate complexes (AR--AD, AD--P450, AR--AD--P450) [26, 27], and/or complexes with more components, as AR--AD--AD--P450, and block the triggering of electron transfer from two- to one-electron mode at the level of AD.
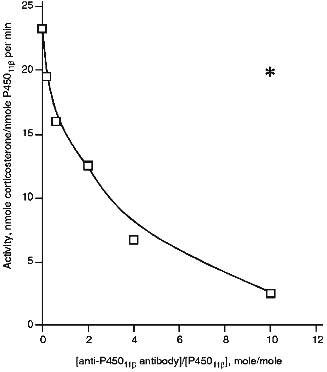
Effect of Anti-P45011beta Antibodies on Activity of Cytochrome P45011beta. Anti-P45011beta antibodies inhibit the hydroxylation of 11beta-deoxycorticosterone in the reconstituted system that is detected by a decreased formation of corticosterone, the product of the reaction (Fig. 1). The degree of inhibition depends on the amount of added antibodies. In the absence of antibodies, the activity is 23 nmoles corticosterone per nmole P45011beta per min, and in the presence of 6-fold excess of antibodies (w/w) over the amount of P45011beta (that is approximately 2-fold molar excess), the activity is inhibited by 44%, decreasing to 12.9 nmoles corticosterone per nmole P45011beta per min.
To test the specificity of inhibition of cytochrome P45011beta-dependent activity by antibodies, we examined the effect of anti-P450scc antibodies on the reaction of hydroxylation of 11beta-deoxycorticosterone under similar conditions at the ratio AR/AD/P45011beta 0.4:5:1. In the presence of 6-fold excess of antibodies, the degree of inhibition was 18%; this is comparable to that observed in the control experiment with non-immune antibodies (15%). Thus, it may be stated that the inhibition of 11beta-hydroxylation by the antibodies to the heme protein is strictly specific.Fig. 1. Activity of cytochrome P45011beta in the presence of anti-P45011beta antibodies. The reconstituted system contained 1 µM cytochrome P45011beta, 5 µM AD, 0.4 µM AR, and 100 µM 11-deoxycorticosterone. The asterisk designates the control activity in the presence of non-immune IgG.
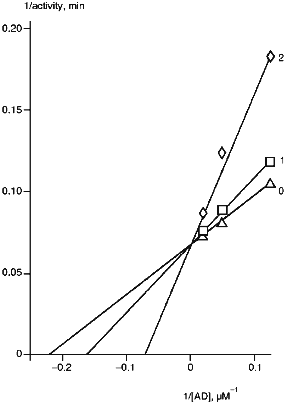
The rates of monooxygenase reactions are significantly dependent on the ratio of cytochrome P450 and intermediate electron carriers [28, 29]. The main factor affecting the reaction rate is the amount of ferredoxin. A 2-fold decrease in AD/P45011beta ratio (from 20:1 to 10:1) correspondingly decreases the reaction rate. In this connection, the effect of AD on corticosterone formation in the presence of anti-P45011beta antibodies was examined (Fig. 2). The strongest inhibition by the antibodies was observed at low AD concentrations. High concentrations of AD prevented inhibition of the reaction, thus suggesting the competition of antibodies and ferredoxin for binding with the same or adjacent regions of cytochrome P45011beta polypeptide sequence.
As follows from our data, the anti-P45011beta antibodies strongly inhibit the reactions of steroid 11beta-hydroxylation; this is in agreement with the other data demonstrating a null effect of anti-P45011beta antibodies on the reactions of 18- and 19-hydroxylation of steroids [30]. However, it follows from our data that anti-P45011beta antibodies may specifically inhibit the reactions of steroid hydroxylation by interfering with the cytochrome P45011beta--ferredoxin interaction.Fig. 2. Dependence of cytochrome P45011beta activity on AD concentration. The reconstituted system contained 0.5 µM cytochrome P45011beta, 0.4 µM AR, and 100 µM 11beta-deoxycorticosterone: 0) in the absence of antibodies; 1, 2) the molar ratios [anti-P45011beta antibody]/[P45011beta] were 0.2 and 0.6, respectively.
Characterization of Antibodies against Polypeptide Fragments of Cytochrome P450scc. Proteolytic modification of cytochrome P450scc in solution by trypsin results in several large polypeptide fragments, thus indicating a domain structure of the heme protein molecule [31]. Native cytochrome P450scc may be cleaved, given a limited time of proteolysis, only in the region Arg250-Asn257, with formation of fragments F1 (29.8 kD) and F2 (26.6 kD). A longer time of proteolysis or increased amount of trypsin result in an additional fragment in the hydrolysate, F3 (16.8 kD according to electrophoresis). Immunofixation electrophoresis of trypsin hydrolysate of cytochrome P450scc in the presence of anti-P450scc antibodies showed a relatively even distribution of antigenic determinants between fragments F1, F2, and F3. These data were prerequisite to the raising of antibodies against these large polypeptide fragments.
To isolate the fragments, native cytochrome P450scc was treated with trypsin at a 50:1 ratio for 30 min (for F1) or 20 min (for F2). F3 was obtained by a 60-min treatment at the cytochrome P450scc/trypsin ratio 10:1. To separate F1 from F2 and F1 from F3, and to remove the non-reacted cytochrome P450scc, thiol-disulfide exchange chromatography on thiopropyl-Sepharose 6B was used. The structural characterization of purified fragments F1 and F2 confirmed their locations in the N- and C-terminal sequences of cytochrome P450scc, respectively. According to amino acid analysis and N-terminal sequence identification, fragments F1 and F2 correspond to the sequences Ile1-Arg256 and Asn257-Ala481, respectively (Tables 2 and 3). The 16.8 kD fragment (according to PAGE) obtained in our experiment is the N-terminal section of fragment F2 (Asn257-Arg399), i.e., it corresponds to fragment F3.
TABLE 2. Amino Acid Composition of
Cytochrome P450 Fragments Used for Immunization
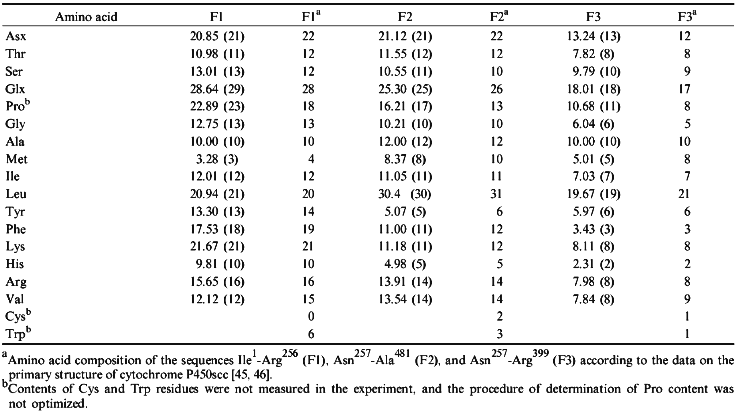
TABLE 3. N-Terminal Amino Acid Sequences of
Cytochrome P450scc Fragments Used for Immunization

When examined by Ouchterlony immunodiffusion, anti-F1 and anti-F2 antibodies do not form precipitates with F2 and F1, respectively, and anti-F2 and anti-F3 antibodies cross-react with F3 and F2. All the antibodies "recognize" cytochrome P450scc, thus indicating the coincidence of antigenic determinants in the fragments and in a full-size molecule of cytochrome P450scc (this does not exclude differences between the whole set of determinants in isolated fragments and that in the corresponding regions of the native heme protein molecule) and suggesting that most determinants are formed by extended regions of the cytochrome P450scc polypeptide chain.
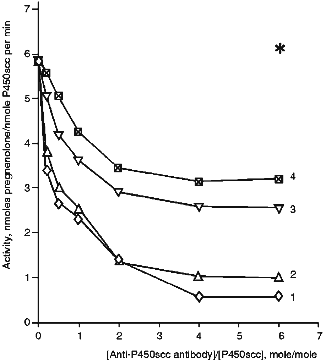
Effects of Antibodies against Cytochrome P450scc and Fragments F1, F2, and F3 on the Heme Protein Activity. In the presence of anti-P450scc antibodies, the cholesterol-hydroxylating activity of cytochrome P450scc in the reconstituted system is inhibited (Fig. 3). With a 2-fold excess of antibodies, the rate of formation of pregnenolone decreases from 5.8 to 1.4 nmoles/nmole P450scc per min (24% of the initial activity). Antibodies raised against the fragments of cytochrome P450scc molecule allow us to assess the functional importance of the domains formed by N- and C-terminal regions of the cytochrome P450scc polypeptide chain. For this purpose, the antibodies against fragments F1, F2, and F3 were examined for their effects on the ability of cytochrome P450scc to convert cholesterol to pregnenolone. As follows from the data in Fig. 3, the antibodies against the fragments of cytochrome P450scc inhibit conversion of cholesterol to pregnenolone. Anti-P450scc and anti-F1 antibodies demonstrate a similar degree of inhibition, and anti-F2 and anti-F3 antibodies have a lower inhibiting effect. Cholesterol conversion to pregnenolone is not completely inhibited. The residual activity comprising up to 10-15% of the initial level may be due both to the presence in the reaction mixture of Tween 20 that aids cholesterol solubilization [32] and affects the aggregate state of the heme protein, and to a possible nonspecific stabilizing effect of the antibodies on the functional state of monooxygenase complexes.
The absence of a marked inhibiting effect of anti-P45011beta antibodies on the reaction of cholesterol hydroxylation proves the earlier conclusion that inhibition of cytochrome P450scc and P45011beta activities by the corresponding antibodies is specific, in spite of some homology in the primary structures of these heme proteins and, probably, the similarity of their ferredoxin-binding sites. It follows from our data that a highly specific character of protein--protein interactions in steroidogenesis is due to the specific three-dimensional structure of mitochondrial cytochromes P450. Addition of antibodies against cytochrome P450scc of bovine adrenocortical mitochondria to the reconstituted system containing cytochrome P450scc of human placental mitochondria and AR and AD of bovine adrenocortical mitochondria does not affect the amount of formed pregnenolone as compared to a similar hybrid system containing a non-immune rabbit serum [33], in spite of immunochemical similarity of the two cytochromes P450scc.Fig. 3. Activity of cytochrome P450scc in the presence of anti-P450scc (1), anti-F1 (2), anti-F2 (3), and anti-F3 (4) antibodies. The reconstituted system contained 1 µM cytochrome P450scc, 4 µM AD, 1 µM AR, and 50 µM cholesterol. The asterisk designates the control activity in the presence of non-immune IgG.
Enzymatic Reduction of Cytochrome P450scc in the Presence of Antibodies. Steroidogenic electron transfer in mitochondria involves the following steps: 1) interaction of AR with NADPH and reduction of the flavoprotein; 2) electron transfer from AR to AD; 3) electron transfer from AD to cytochromes P450scc or P45011beta. The last step is the most complex and involves two subsequent electron transfers; this suggests at least two stages of reduction of the heme protein AD [34]. Interaction of AD with cytochrome P450scc depends on the presence of the substrate in the active center [35] and is coupled to the successive hydroxylations of intermediates resulting in formation of pregnenolone.
To determine the step of cholesterol hydroxylation which may be affected by the antibodies, we examined the effects of anti-P450scc antibodies and antibodies against the heme protein fragments on individual steps of monooxygenase catalysis, including one step of electron transfer, the enzymatic reduction of cytochrome P450 by the first electron in solution in the presence of NADPH, AR, and AD. Preliminary experiments have shown that addition of anti-P450scc antibodies does not affect the rate of formation of carbon monoxide complex of cytochrome P450scc reduced by sodium dithionite.
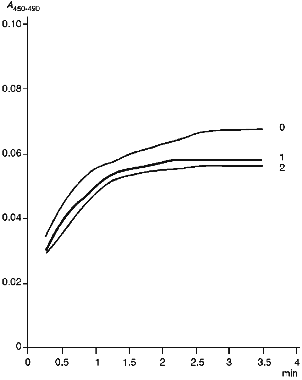
Figure 4 gives kinetic curves of enzymatic reduction of cytochrome P450scc in the absence and in the presence of anti-P450scc antibodies. As follows from the data, the antibodies have no significant effect on the rate of enzymatic reduction of cytochrome P450scc, though they slightly decrease (by 10%) the amount of enzymatically reduced heme protein. As shown in the experiments on chemical reduction of cytochrome P450scc run in parallel, this decrease correlates with accumulation of cytochrome P420 in the system containing AR, AD, cytochrome P450scc and non-immune IgG. Thus, anti-P450scc antibodies at least exert no significant specific effect on the enzymatic reduction of cytochrome P450scc by the first electron.
Examination of Interactions of Cytochrome P450scc with Cholesterol and AD in the Presence of Antibodies. The mechanism of the inhibitory action of the antibodies is complex and involves both the interaction with functionally active sites of antigen molecules and conformational rearrangements of the protein molecule. The antibodies do not affect chemical and enzymatic reduction of cytochrome P450scc. In addition, as shown for cytochrome P45011beta, the inhibiting effect of specific antibodies depends on the heme protein/AD ratio. All these data suggest that antibodies disturb electron transfer by interfering with AD--P450scc interaction. However, an effect of the antibodies on the binding of cholesterol in the active center of cytochrome P450scc can not be excluded. To test the last suggestion, we studied the interaction of a substrate-free low-spin form of cytochrome P450scc in which the heme protein is in a low-spin state with cholesterol. Addition of cholesterol result in transition of cytochrome P450scc to the high-spin state detected by the difference spectrum with maximum at 390 nm and minimum at 420 nm. Addition of anti-P450scc antibodies does not change the character of the interaction as compared to this process in the presence of non-immune antibodies. The amplitude of spectral change slightly decreases as compared to the control in the absence of any antibodies, and this may be due to a nonspecific adsorption of cholesterol on IgG. The antibodies against the fragments of cytochrome P450scc molecule also cause no significant changes in the interaction of the heme protein with the substrate.Fig. 4. Formation of carbon monoxide complex during enzymatic reduction of cytochrome P450scc: 0) in the absence of antibodies; 1) in the presence of non-immune IgG (at 4-fold molar excess over cytochrome P450scc content); 2) in the presence of an equal amount of anti-P450scc antibodies.
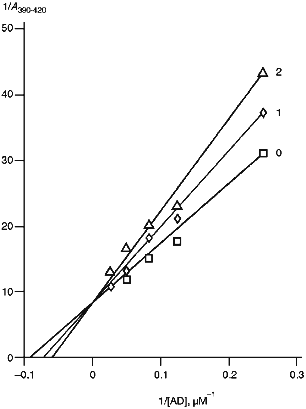
To examine the interaction of cytochrome P450scc with AD, we used spectrophotometric titration of the low-spin form of cytochrome P450scc resulting from addition of Tween 20 to the heme protein by ferredoxin. AD is known to be a high-spin effector of cytochrome P450scc [36]. As follows from the data in Fig. 5 and Table 4, in the presence of antibodies against cytochrome P450scc and its three polypeptide fragments, the amplitude of spectral changes characteristic of the spin transition decreases, and the character of these changes (increased apparent Ks and unchanged Amax) indicates the competition of the antibodies and AD for binding with cytochrome P450scc. However, addition of non-immune rabbit antibodies does not change the character of the spin transition of the heme protein, thus indicating a specific character of the effects of anti-P450scc antibodies and antibodies against its polypeptide fragments on the interaction of AD with the heme protein. The experiments performed prove the suggestion that in the case with cytochrome P450scc, antibodies can selectively destroy the electron-transfer chain by blocking the interaction of AD with the heme protein. However, given the excess of reagents, the first electron is transferred with no significant decrease in reaction rate.
TABLE 4. Effects of Anti-P450scc, Anti-F1, Anti-F2, and Anti-F3 Antibodies on Kinetic Parameters of Interaction of Cytochrome P450scc with AD and on Its Activity at Different AD ConcentrationsaFig. 5. Dependence of the spectrophotometric changes in A390-420 of cytochrome P450scc low-spin form on AD concentration: 0) in the absence of antibodies; 1, 2) at the ratios [Anti-P450scc antibody]/[P450scc] (mole/mole) 2 and 4, respectively.
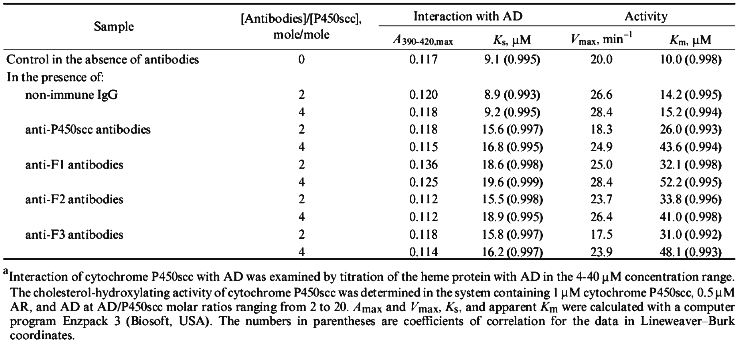
To find out to what extent the ratio of AD and antibodies influence the effect exerted by antibodies on the final steps of the monooxygenase cycle, we determined the dependence of enzymatic activity of cytochrome P450scc on AD concentration in the reconstituted system in the presence of antibodies. As follows from the data in Table 4, the addition of specific antibodies results in 2-3.5-fold increase of Km of the process as compared to the activity in the presence of non-immune antibodies. The calculated Vmax, as Amax on spectrophotometric titration of cytochrome P450scc by AD undergoes no significant changes and reaches its usual values after addition of an excess of ferredoxin. These data agree with those obtained for cytochrome P45011beta (Fig. 1), where the degree of inhibition of enzymatic activity of the heme protein by anti-P45011beta antibodies also depends on the ratio AD/P45011beta (for large molar excesses of AD the effect of antibodies is not observed), and indicate that antibodies inhibit the interaction of mitochondrial cytochromes P450 with ferredoxin by a general mechanism.
DISCUSSION
The present data indicate that antibodies against mitochondrial cytochromes P450scc and P45011beta specifically inhibit the activity of the heme proteins in the reconstituted system by 90%. In addition, our data lead to several important conclusions concerning structural--functional interactions in monooxygenase complexes. The inhibition of cholesterol conversion to pregnenolone by antibodies against cytochrome P450scc indicates that during the monooxygenase cycle, in which the cholesterol molecule is at least thrice attacked by activated molecular oxygen, both the functional domains of the heme protein molecule formed by fragments F1 and F2 participate in enzymatic reaction. The latter demonstrates a close relationship between individual sites of the cytochrome P450scc polypeptide chain in monooxygenase catalysis and disagrees with the model of membrane organization, according to which one part of the heme protein represents a catalytic domain, and the other one fulfills the function of immobilization of the protein in the phospholipid membrane, which is a characteristic of cytochrome b5 [37-39] and NADPH-cytochrome P450-reductase [40, 41] and is also suggested for microsomal cytochromes P450 [42-44].
It is important to elucidate the mechanism of the inhibiting effect of the antibodies. The present data indicate that the antibodies may disturb the electron transfer between AD and cytochromes P450scc and P45011beta, and, therefore, the effect of the antibodies is exerted at the level of protein--protein interactions. This suggests that the antigenic determinants of cytochrome P450scc and the sites responsible for the interaction with AD in a heme protein molecule either coincide or at least partly overlap. Our data allow us to conclude that the AD-binding region of cytochrome P450scc is probably organized by several sites of the polypeptide chain located in two large domains of the heme protein formed by fragments F1 and F2, because the antibodies against these fragments and against fragment F3, as well as the antibodies against the whole molecule of cytochrome P450scc, are able to interfere with heme protein--AD interaction.
LITERATURE CITED
1.Shkumatov, V. M., Usanov, S. A., Chashchin, V. L.,
and Akhrem, A. A. (1985) Pharmazie, 40, 757-766.
2.Usanov, S. A., Chashchin, V. L., and Akhrem, A. A.
(1990) in Molecular Mechanisms of Adrenal Steroidogenesis and
Aspects of Regulations and Application (Ruckpaul, K., and Rein, H.,
eds.) Frontiers in Biotransformation, Vol. 3, Akademie-Verlag,
Berlin, pp. 1-57.
3.Thummel, K. E., Favreau, L. V., Mole, J. E., and
Shenkman, J. B. (1988) Arch. Biochem Biophys., 266,
319-333.
4.Kikuta, Y., Kukunose, E., Okumoto, T., Kubota, I.,
and Kukunose, M. (1990) J. Biochem., 107, 280-286.
5.Ding, X., and Coon, M. (1988)
Biochemistry, 27, 8330-8337.
6.Leo, M. A., Lasker, J. M., Raucy, J. L., Kim, C.,
Black, M., and Lieber, C. S. (1989) Arch. Biochem. Biophys.,
269, 306-312.
7.Park, S. S., Waxman, D. L., Lapeson, D. P.,
Shenkman, J. B., and Gelboin, H. V. (1988) Biochem.
Pharmacol., 38, 3067-3074.
8.LeProvost, E., Flinois, J. P., Beaune, P., and
Leroux, J. P. (1981) Biochem. Biophys. Res. Commun., 101,
547-554.
9.Nakajima, T., Elovaara, E., Park, S. S., Gelboin,
H., Hietanen, E., and Vainio, H. (1989) Carcinogenesis,
10, 11713-1717.
10.Knan, W. A., Park, S. S., Gelboin, H. V.,
Bickers, D. R., and Mukhtar, N. (1989) J. Pharmacol. Exp.
Therap., 249, 921-927.
11.Perrot, N., Nalpas, B., Yang, C. S., and Beaune,
P. H. (1989) Europ. J. Clin. Invest., 41, 1735-1743.
12.Ekstrom, G., von Bahr, C., and Ingelman-Sundberg,
M. (1989) Biochem. Pharmacol., 38, 689-693.
13.Schweizer, M., Peter, M. A., Filipovic, D.,
Tinner, R., Bosshard, H. R., and Oertle, M. (1991) Arch. Biochem.
Biophys., 288, 64-70.
14.Dedinsky, I. R., Kozin, S. A., and Archakov, A.
I. (1994) in Cytochrome P450. Biochemistry, Biophysics, and
Molecular Biology (Lechner, M. C., ed.) John Libbey Eurotext,
Paris, pp. 603-610.
15.Shen, S., and Strobel, H. W. (1994)
Biochemistry, 33, 8807-8812.
16.Dus, K. M., Litchfield, H. J., Hippenmeyer, P.
J., Bumpus, J. A., Obidoa, O., Spitsberg, V., and Jefcoate, C.
(1980) Eur. J. Biochem., 111, 307-314.
17.Kashiwagi, K., McDonald, A. B., and Salhanick, H.
A. (1982) J. Biol. Chem., 257, 2212-2217.
18.Usanov, S. A., Turko, I. V., Akhrem, A. A., and
Chashchin, V. L. (1984) Biokhimiya, 49, 1810-1818.
19.Usanov, S. A., Chernogolov, A. A., Akhrem, A. A.,
and Chashchin, V. L. (1987) Biokhimiya, 52, 110-122.
20.Usanov, S. A., Chernogolov, A. A., and Chashchin,
V. L. (1989) FEBS Lett., 255, 125-128.
21.Usanov, S. A., Pikuleva, I. A., Chashchin, V. L.,
and Akhrem, A. A. (1984) Bioorg. Khim., 10, 32-45.
22.Martsev, S. P., Chashchin, V. L. and Akhrem, A.
A. (1985) Biokhimiya, 50, 243-257.
23.Omura, T., and Sato, R. (1964) J. Biol.
Chem., 239, 2370-2378.
24.Phillips, A. P., Martin, K. L., and Horton, W. H.
(1984) J. Immunol. Meth., 74, 385-393.
25.Schenkman, J. B. (1970) Biochemistry,
9, 2081-2091.
26.Usanov, S. A., Turko, I. V., Chashchin, V. L.,
and Akhrem, A. A. (1985) Biochim. Biophys. Acta, 832,
288-296.
27.Turko, I. V., Adamovich, T. B., Kirillova, N. M.,
Usanov, S. A., and Chashchin, V. L. (1989) Biochim. Biophys.
Acta, 996, 37-42.
28.Seybert, D. W., Lambeth, Y. D., and Kamin, H.
(1978) J. Biol. Chem., 253, 8355-8358.
29.Akhrem, A. A., Vasilevskii, V. I., Shkumatov, V.
M., and Chashchin, V. L. (1980) Bioorg. Khim., 6,
400-412.
30.Suhara, K., Gomi, T., Sato, H., Itagaki, E.,
Takemori, S., and Katagari, M. (1978) Arch. Biochem.
Biophys., 190, 290-299.
31.Chashchin, V. L., Shkumatov, V. M., Usanov, S.
A., Vasilevskii, V. I., Turko, I. V., and Akhrem, A. A. (1985)
Biomed. Biochim. Acta, 44, 665-677.
32.Takikawa, O., Gomi, T., Suhara, K., Itagaki, E.,
Takemori, S., and Katagiri, M. (1978) Arch. Biochem. Biphys.,
190, 300-306.
33.Usanov, S. A., Honkakoski, P., Lang, M. A.,
Pasanen, M., Pelkonen, O., and Raunio, H. (1989) Biochim. Biophys.
Acta, 998, 185-195.
34.Lambeth, J. D., Seybert, D. W., and Kamin, H.
(1979) J. Biol. Chem., 254, 7255-7264.
35.Kido, T., Arakawa, M., and Kimura, T. (1979)
J. Biol. Chem., 254, 8377-8385.
36.Katagiri, M., Takikawa, O., Sato, H., and Suhara,
K. (1977) Biochem. Biophys. Res. Commun., 72,
804-809.
37.Spatz, L., and Strittmatter, P. (1971) Proc.
Natl. Acad. Sci. USA, 68, 1042-1046.
38.Abe, K., Kimura, S., Kizawa, R., Anan, F. K., and
Sugita, Y. (1985) J. Biochem., 97, 1659-1668.
39.Ozols, J. (1989) Biochim. Biophys.
Acta, 997, 121-130.
40.Black, S. D., and Coon, M. J. (1982) J. Biol.
Chem., 257, 5929-5938.
41.Nisimoto, Y., and Lambeth, J. D. (1985) Arch.
Biochem. Biophys., 241, 386-396.
42.Nelson, D. R., and Strobel, H. W. (1988) J.
Biol. Chem., 263, 6038-6050.
43.Tretyakov, V. E., Degtyarenko, K. N., Uvarov, V.
Yu., and Archakov, A. I. (1989) Mol. Biol. (Moscow), 23,
1322-1331.
44.Sakaguchi, M., Mihara, K., and Omura, T. (1994)
in Cytochrome P450. Biochemistry, Biophysics, and Molecular
Biology (Lechner, M. C., ed.) John Libbey Eurotext, Paris, pp.
603-610.
45.Chashchin, V. L., Lapko, V. N., Adamovich, T. B.,
Lapko, A. G., Kuprina, N. S., Kirillova, N. M., Berikbaeva, T. M.,
Akhrem, A. A., and Zolotarev, A. I. (1985) Bioorg. Khim.,
11, 1048-1067.
46.Morohashi, K., Yoshioka, H., Gotoh, O., Okada,
K., Yamamoto, K., Miyata, T., Sogawa., K., Fujii-Kuriyama, Y., and
Omura, T. (1984) Proc. Natl. Acad. Sci. USA, 81,
4647-4651.