Hypochlorite Destroys Carotenoids in Low Density Lipoproteins Thus Decreasing Their Resistance to Peroxidative Modification
O. M. Panasenko,1,2 O. O. Panasenko,3 K. Briviba,4 and H. Sies4
1Institute of Physico-Chemical Medicine, ul. M. Pirogovskaya 1a, Moscow, 119828 Russia; E-mail: panasenko@glas.apc.org2To whom correspondence should be addressed.
3Department of Biochemistry, School of Biology, Lomonosov Moscow State University, Moscow, 119899 Russia; fax: (095) 939-3955.
4Institute of Physiological Chemistry 1, Heinrich Heine University Dusseldorf, 101007, D-40001, Dusseldorf, Germany; fax: (+49) 211-811-3029.
Submitted June 10, 1997.
The effects of hypochlorite (HOCl/OCl-) on the content of carotenoids (trans-lycopene, 5-cis-lycopene, alpha- and beta-carotene) and oxycarotenoids (lutein, zeaxanthin, trans- and cis-2´,3´-anhydrolutein, alpha- and beta-cryptoxanthin) in human blood low-density lipoproteins (LDL) were compared using HPLC. Hypochlorite decreased the content of all the above-mentioned pigments in LDL. However, it was more reactive towards carotenoids rather than to their oxy derivatives. The ability of carotenoids and oxycarotenoids to scavenge HOCl/OCl- decreases in the series: trans-lycopene ~ 5-lycopene > alpha-carotene > beta-carotene > zeaxanthin > alpha-cryptoxanthin > cis-2´,3´-anhydrolutein > beta-cryptoxanthin > trans-2´,3´-anhydrolutein > lutein. Preincubation of LDL with hypochlorite decreased their resistance to Cu2+-induced accumulation of dienic conjugates that are produced in the course of lipid peroxidation. The data suggest that hypochlorite-induced destruction of carotenoids in LDL decreases their resistance to oxidative modification, thus promoting the development of early stages of atherosclerosis.
KEY WORDS: hypochlorite, low-density lipoproteins (LDL), carotenoids, oxycarotenoids, xanthophylls, lipid peroxidation, antioxidants, atherosclerosis.
Carotenoids are pigments widely spread in nature. They play a key role in the defense of a cell against lesions that occur during photosensitization both in vitro and in vivo [1]. This property of carotenoids is ascribed to their antioxidant activity. Actually, beta-carotene and other carotenoids as well as their oxy derivatives (xanthophylls) efficiently react with oxygen [1, 2], peroxyl, and other radicals at the stage of reaction initiation and chain branching [1, 3-6]. The breaking of chain reaction inhibits free-radical reactions including lipid peroxidation (LPO) [7]. beta-Carotene exerts a synergistic effect, enhancing the ability of alpha-tocopherol to scavenge free radicals and thus increasing its antioxidative reactivity [8]. Finally, it is known that carotenoids can trap and quench singlet oxygen [9, 10], a well-known inducer of free-radical LPO reactions [11].
Carotenoids in human blood are associated predominantly with lipoproteins (mainly with low-density lipoproteins (LDL)) [12]. It is known that oxidative modification of LDL disrupts their lipid-transporting function [13-15]. This may result in the development of pathological states, atherosclerosis in particular [15]. One possible reason for LDL oxidative destruction is their interaction with hypochlorous acid (HOCl) or its dissociated form, hypochlorite (OCl-) [16-19], which are produced in vivo by activated neutrophils and monocytic macrophages in myeloperoxidase-catalyzed reaction [20, 21]:
In this study, we investigated the effect of different concentrations of hypochlorite on the content of the most important carotenoids (i.e., trans-lycopene, 5-cis-lycopene, alpha- and beta-carotene) and oxycarotenoids (lutein, zeaxanthin, trans- and cis-2´,3´-anhydrolutein, as well as alpha- and beta-cryptoxanthin) contained in LDL. We also evaluated the ability of hypochlorite to affect LDL resistance to free-radical lipid peroxidation.
MATERIALS AND METHODS
Isolation of Lipoproteins. Low-density lipoproteins were isolated from the blood of healthy donors by preparative centrifugation in NaBr solutions of specified density; these contained 0.01% EDTA, with preliminary flotation of lipoproteins of very low density [24]. The obtained LDL were dialyzed at 4°C for 20 h against 10 mM phosphate buffer (pH 7.4) containing 145 mM NaCl, filtered through a 0.2-µm filter, and stored at 4°C. The concentration of LDL was determined by measuring the concentration of protein contained in them [25].
Incubation of LDL with Hypochlorite and Determination of Carotenoids. Low-density lipoproteins (0.6 mg protein per ml) were incubated with different concentrations of hypochlorite at 25°C for 10 min in medium containing 145 mM NaCl and 100 mM phosphate buffer (pH 7.4). The mixture (1 ml) was then diluted into 1 ml of ethanol and 1.5 ml of hexane--dichloromethane mixture (5:1 v/v) and then stirred thoroughly. Aqueous and organic phases were segregated by centrifuging at 1500g for 5 min. The upper phase (1 ml) was evaporated under a nitrogen flow, diluted into 100 µl of a mixture containing methanol--acetonitrile--2-propanediol (54:44:2 v/v), and then used in chromatographic analysis.
Carotenoids and oxycarotenoids in the specimens were analyzed by HPLC on a 5-µm Suplex pKb 100 column (250 × 4.6 mm) (Supelco, USA) supplemented with a 5-µm Suplex pKb 100 precolumn (20 × 4.6 mm) (Supelco). The elution rate was 1 ml/min. The compounds were detected spectrophotometrically at 450 nm. Mixtures A containing methanol--acetonitrile--2-propanol (54:44:2 v/v) and B containing mixture A and H2O (85:15 v/v) were used as eluents. Optimal separation of oxycarotenoids was reached using the following gradient: 64% A and 36% B (0-5 min), 64% --> 100% A and 36% --> 0% B (5-15 min), 100% A (15-27 min), 64% A and 36% B (27-32 min) [26, 27].
Cu2+-Induced Oxidation of LDL. Low-density lipoproteins (66 µg protein per ml) were preincubated at 25°C for 40 min with different concentrations of hypochlorite (0, 0.05, 0.1, 0.2, and 0.3 mM) in 10 mM phosphate buffer (pH 7.4) containing 145 mM NaCl. In preliminary experiments, we found that this time is required for hypochlorite to react completely with LDL components. Thereafter, we placed the specimens into quartz spectrophotometric cuvettes, added 10 µM CuSO4·5H2O, and recorded the optical density at 234 nm (maximal absorbance of dienic conjugates that are LPO products [28]) with 5-min intervals. The optical density was measured simultaneously in 5 cuvettes.
The concentration of hypochlorite in the stock solution was determined at pH 12 by measuring its absorbance at 290 nm, given that its molar extinction coefficient epsilon290 is 350 M-1·cm-1 [29].
Reagents. Carotenoids and oxycarotenoids were determined using lutein, zeaxanthin, trans-2´,3´-anhydrolutein, cis-2´,3´-anhydrolutein, alpha-cryptoxanthin, beta-cryptoxanthin, trans-lycopene, 5-cis-lycopene, alpha-carotene, beta-carotene, 9-cis-beta-carotene, and 13-cis-beta-carotene as standards. These reagents were obtained from Hoffmann-La Roche (Switzerland). The other reagents and solvents used in chromatographic analysis were from Merck (Germany).
RESULTS
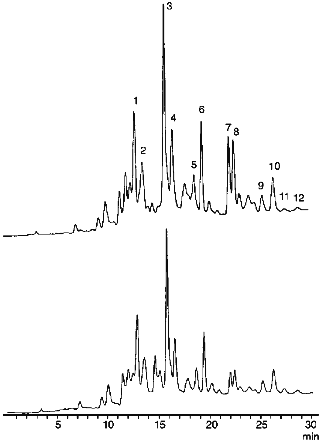
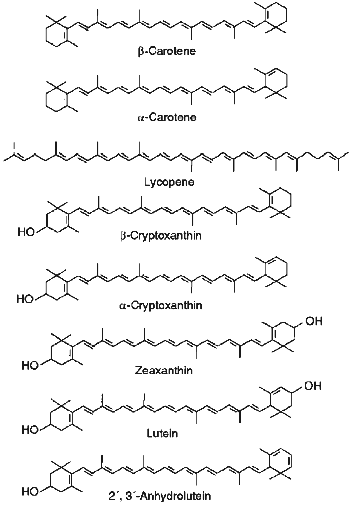
Effect of Hypochlorite on Carotenoid Content in LDL. Figure 1 shows typical chromatograms of the extracts of native LDL (above) and those incubated with hypochlorite (below). We identified all essential carotenoids (i.e., trans-lycopene, 5-cis-lycopene, alpha-carotene, trans-beta-carotene, 9-cis-beta-carotene, and 13-cis-beta-carotene) and their oxy derivatives (i.e., lutein, zeaxanthin, trans- and cis-2´,3´-anhydrolutein, as well as alpha- and beta-cryptoxanthin) that could be usually detected in plasma [9, 12]. Structural formulas of trans-isomers of the listed carotenoids are shown in Fig. 2. As seen from Fig. 1, incubation of LDL with 0.2 mM hypochlorite reduces the magnitude of all the peaks detected. However, the value of this reduction varies significantly for different carotenoids.
Fig. 1. Chromatographic profiles of extracts of native LDL (above) and those incubated with 0.2 mM hypochlorite at 25°C for 10 min (below). Identified components include lutein (1), zeaxanthin (2), trans-2',3'-anhydrolutein (3), cis-2´,3´-anhydrolutein (4), alpha-cryptoxanthin (5), beta-cryptoxanthin (6), trans-lycopene (7), 5-cis-lycopene (8), alpha-carotene (9), beta-carotene (10), 9-cis-beta-carotene (11), and 13-cis-beta-carotene (12). The incubation medium contained 145 mM NaCl and 100 mM phosphate buffer (pH 7.4). The protein concentration in the LDL was 0.6 mg/ml.
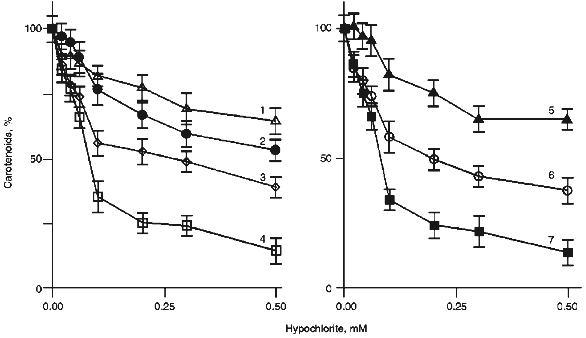
To compare the ability of carotenoids to react with HOCl/OCl-, we determined their content in LDL after preincubation of the latter with different concentrations of hypochlorite. Several curves illustrating the dependence of the pigment content in LDL on the concentration of hypochlorite in the incubation medium are shown in Fig. 3. Similar patterns were obtained for all carotenoids and oxycarotenoids analyzed. A 10-min incubation of LDL with 0.1 mM hypochlorite decreased the lycopene level by 66 ± 4% (Fig. 3, curve 7), whereas the content of trans-2´,3´-anhydrolutein decreased by 18 ± 6% (Fig. 3, curve 5).Fig. 2. Structural formulas of trans-isomers of carotenoids and oxycarotenoids analyzed in this study.
The concentration of hypochlorite in a 10-min incubation which resulted in a 25% decrease in the carotenoid content in LDL (CHOCl-25), served as a reference parameter to compare the ability of LDL-associated carotenoids to scavenge hypochlorite. The calculated values of CHOCl-25 for all studied carotenoids are shown in increasing order in Table 1.Fig. 3. Dependence of the content of lutein (1), beta-cryptoxanthin (2), alpha-carotene (3), 5-cis-lycopene (4), trans-2´,3´-anhydrolutein (5), beta-carotene (6), and trans-lycopene (7) in LDL on the concentration of hypochlorite in the incubation medium. The incubation conditions are the same as those indicated in the legend to Fig. 1. The content of carotenoids measured in the absence of hypochlorite was taken as 100%.
TABLE 1. Concentration of Hypochlorite
Causing a 25% Decrease in Carotenoid and Oxycarotenoid Content in LDL
(CHOCl-25)
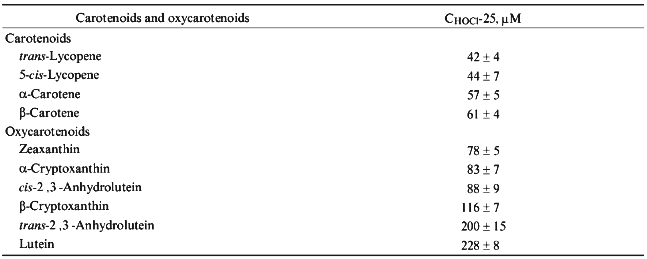
Interestingly, carotenoids appeared to be more reactive towards hypochlorite than oxycarotenoids. All of them are grouped in the upper part of Table 1. Lycopene is the most efficient scavenger of hypochlorite. In contrast, oxycarotenoids (xanthophylls) are grouped in the lower part of Table 1. Lutein was the least reactive towards hypochlorite of all compounds studied.
Effect of Hypochlorite on the Resistance of LDL to Cu2+-Induced LPO. To study the resistance of LDL-associated lipids to peroxidation, we used discontinuous monitoring of the optical density at 234 nm (corresponding to maximal absorbance of dienic conjugates) in the course of their Cu2+-induced oxidation [28]. This approach allows simultaneous characterization of the ability of LDL to undergo oxidation using several parameters.
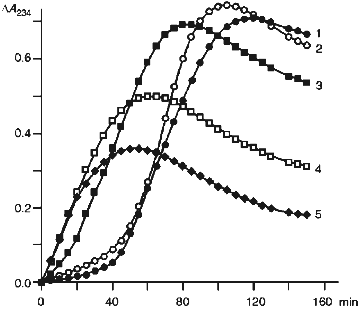
Kinetics of the changes in optical density at 234 nm occurring in the suspension of native LDL (1) and those pretreated with hypochlorite (2-5) during their incubation with Cu2+ is shown in Fig. 4. Curve 1 is sigmoidal with a pronounced induction period; its duration characterizes LDL antioxidative potential (i.e., antioxidant content). The maximal slope of the curve reflects the oxidation rate, while the maximal value of optical density corresponds to the absolute level of dienic conjugates accumulated in LDL [28].
The data present in Fig. 4 indicate that a 40-min preincubation of LDL with 50 µM hypochlorite (curve 2) somewhat reduces the induction period and increases the maximal slope of the curve as well as the maximal value of optical density. Taken together, these changes are indicative of a decrease in LDL resistance to Cu2+-induced oxidation. A further increase in the concentration of hypochlorite in the preincubation medium results in a pronounced decrease in duration of the induction period. For instance, as is shown in Fig. 4 (curve 3), 0.1 mM hypochlorite causes at least fivefold reduction of the induction period. Moreover, induction period is completely absent from curves 4 and 5 (Fig. 4), which were obtained in the presence of 0.2 and 0.3 mM hypochlorite, respectively. This finding suggests that after preincubation with hypochlorite at concentrations no less than 0.2 mM, LDL virtually loose their antioxidative potential.Fig. 4. Changes in the optical density measured at 234 nm in the suspension of native LDL (1) and those preincubated with 50 (2), 100 (3), 200 (4), and 300 (5) µM hypochlorite in the course of their incubation with Cu2+. LDL (66 µg protein per ml) were incubated at 25°C for 40 min with the specified concentrations of hypochlorite. The mixture was then placed into the spectrophotometric cuvettes and the reaction was started by addition of 10 µM CuSO4·5H2O. The reaction rate was monitored at 234 nm at 30°C. The reaction medium composition was the same as that indicated in the legend to Fig. 1.
The observed decrease of the maximal value of optical density occurring on increase in hypochlorite concentration (Fig. 4) can be accounted for by the known ability of the latter to react with unsaturated bonds in fatty acids [30]. The increase in hypochlorite concentration in such a reaction sharply decreases the total amount of lipids containing unsaturated fatty acids, which are potential substrates in reactions yielding dienic conjugates.
DISCUSSION
It is known that hypochlorite produced by neutrophils and other phagocytes during their activation is a potent oxidant and chlorinating agent and, therefore, can enter reactions with apolipoproteins (such as oxidation of SH-groups, chlorination of NH2-groups, and destruction of aromatic and unsaturated amino acid residues) [19, 31, 32], phospholipids [30, 33], free fatty acids [30], as well as cholesterol and its esters [33, 34], which are included in the composition of human blood lipoproteins. The interaction of hypochlorite with lipids containing unsaturated fatty acids is attended by accumulation of primary (lipid hydroperoxides and dienic conjugates), secondary (aldehydes reacting with 2-thiobarbituric acid), and final (fluorescent Schiff bases) LPO products [16-18]. All these processes result in modifications of structural organization of human blood lipoproteins, which manifest themselves as disturbances in the dynamics of the lipid and protein phases of lipoproteins and alteration of their surface potential. As a consequence, they entail disruption of the lipid-transporting function of lipoproteins [35, 36]. Such a modification imparts atherogenic properties to lipoproteins (LDL, in particular), thus being an important trait of development of early stages of atherosclerosis [13, 15].
The ability of LDL to undergo oxidative modification depends primarily on their antioxidative potential that is due mainly to the content of so-called lipid-soluble antioxidants (i.e., alpha- and gamma-tocopherol, ubiquinol-10, and different carotenoids) [37, 38].
In this study we compared the ability of carotenoids (trans-lycopene, 5-cis-lycopene, alpha- and beta-carotene) and their oxy derivatives (lutein, zeaxanthin, trans- and cis-2´,3´-anhydrolutein, as well as alpha- and beta-cryptoxanthin) which are included in the composition of LDL to react with hypochlorite. The results significantly broaden the list of known hypochlorite scavengers. We discovered that trans- and cis-isomers of lycopene as well as alpha- and beta-carotene scavenged hypochlorite the most readily. Interestingly, they range up to 50% of all carotenoids in human blood [26]. The study of the distribution of these carotenoids between the main classes of lipoproteins showed that LDL contain 73, 67, and 58% of lycopene, beta- and alpha-carotene, respectively [12].
Oxycarotenoids contained in LDL reacted with hypochlorite less efficiently. For example, as shown in Table 1, the content of lutein in LDL decreased by 25% in the presence of the concentration of hypochlorite 5.4 times higher than that in the case of trans-lycopene. It is interesting that the content of oxycarotenoids in LDL is not high: low-density lipoproteins contain only 31% of lutein and zeaxanthin, whereas high-density lipoproteins contain 53% of these carotenoids [12]. Thus, LDL contain predominantly more potent scavengers of hypochlorite (namely, lycopene, beta-carotene, and alpha-carotene), while their oxy derivatives, which less efficiently react with hypochlorite, are distributed predominantly between high-density and very-low-density lipoproteins [12]. It cannot be excluded that such a distribution of antioxidants among different classes of lipoproteins in human blood is a manifestation of a natural protective reaction of the organism against oxidative modification of LDL.
Lycopene, that was known so far as the most efficient quencher of singlet oxygen [9], free radical scavenger [6], and LPO inhibitor [37], also proved to be the most efficient scavenger of hypochlorite among the carotenoids.
With regard for the high reactivity of hypochlorite towards carotenoids, we suggest that incubation of LDL with hypochlorite should decrease their antioxidative potential. Actually, as seen from Fig. 4, hypochlorite at concentrations close to physiological [39] markedly reduces the induction period of Cu2+-induced accumulation of dienic conjugates in LDL. This finding indicates a decreased resistance of hypochlorite-treated LDL to free-radical LPO reactions and, therefore, a decrease in their antioxidative potential.
The results suggest that the increase in the ability of LDL to undergo lipid peroxidation may be due, at least in part, to hypochlorite-induced degradation of carotenoids.
It is known that carotenoids not only protect lipoproteins against oxidative modification [37], but also inhibit the development of atherosclerosis in rabbits [40]. A possible explanation of this phenomenon is the ability of carotenoids to efficiently scavenge hypochlorite.
This study was supported by the Alexander von Humboldt Foundation (Germany).
LITERATURE CITED
1.Palozza, P., and Krinsky, N. I. (1992) Meth.
Enzymol., 213, 403-420.
2.Weitberg, A. B., Weitzman, S. A., Clark, E. P., and
Stossel, T. P. (1985) J. Clin. Invest., 75,
1835-1841.
3.Krinsky, N. I. (1989) Free Radical Biol.
Med., 7, 617-635.
4.Burton, G. W., and Ingold, K. U. (1984)
Science, 224, 569-573.
5.Burton, G. W. (1989) J. Nutrition,
119, 109-111.
6.Miller, N. J., Sampson, J., Candeias, L. P.
Bramley, P. M., and Rice-Evance, C. A. (1996) FEBS Lett.,
384, 240-242.
7.Palozza, P., and Krinsky, N. I. (1992) Arch.
Biochem. Biophys., 297, 291-295.
8.Palozza, P., and Krinsky, N. I. (1992) Arch.
Biochem. Biophys., 297, 184-187.
9.Sies, H., Stahl, W., and Sundqoist, A. R. (1992)
Ann. N. Y. Acad. Sci. USA, 669, 7-22.
10.Stahl, W., Nikolai, S., Briviba, K., Hanusch, M.,
Broszeit, G., Paters, M., Martin, H.-D., and Sies, H. (1997)
Carcinogenesis, 18, 89-92.
11.Frankel, E. N. (1987) Chem. Phys. Lipids,
44, 73-85.
12.Clevidence, B. A., and Bieri, J. G. (1993)
Meth. Enzymol., 214, 33-46.
13.Panasenko, O. M., and Sergienko, V. I. (1993)
Biol. Membr. (Moscow), 10, 341-382.
14.Panasenko, O. M., Vol'nova, T. V., Azizova, O.
A., and Vladimirov, Yu. A. (1988) Byull. Eksp. Biol. Med.,
106, 277-280.
15.Berliner, J. A., and Heinecke, J. W. (1996)
Free Radical Biol. Med., 20, 707-727.
16.Evgina, S. A., Panasenko, O. M., Sergienko, V.
I., and Vladimirov, Yu. A. (1992) Biol. Membr. (Moscow),
9, 946-953.
17.Panasenko, O. M., Evgina, S. A., Aidyraliev, R.
K., Sergienko, V. I., and Vladimirov, Yu. A. (1994) Free Radical
Biol. Med., 16, 143-148.
18.Panasenko, O. M., Evgina, S. A., Driomina, E. S.,
Sharov, V. S., Sergienko, V. I., and Vladimirov, Yu. A. (1995) Free
Radical Biol. Med., 19, 133-140.
19.Hazell, L. J., and Stocker, R. (1993) Biochem.
J., 290, 165-172.
20.Edwards, S. W. (1994) Biochemistry and
Physiology of the Neutrophil, University Press, Cambridge, pp.
149-187.
21.Mayanskii, A. N., and Mayanskii, D. N. (1985)
Essays on Neutrophil and Macrophage [in Russian], Nauka,
Novosibirsk.
22.Esterbauer, H., Dieber-Rotheneder, M., Striegl,
G., and Waeg, G. (1991) Am. J. Clin. Nutr., 53,
314S.
23.Winkhofer-Roob, B. M., Puhl, H., Khoschsorur, G.,
Van't Hof, M. A., Esterbauer, H., and Shmerling, D. H. (1995) Free
Radical Biol. Med., 18, 848-859.
24.Schumaker, V. N., and Puppione, L. (1986)
Meth. Enzymol., 128, 155-171.
25.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and
Randall, R. J. (1951) J. Biol. Chem., 193, 265-275.
26.Stahl, W., and Sies, H. (1994) Meth.
Enzymol., 234, 388-400.
27.Gaertner, C., Stahl, W., and Sies, H. (1997)
Am. J. Clin. Nutr., (in press).
28.Esterbauer, H., Striegl, G., Puhl, H., and
Rotheneder, M. (1989) Free Radical Res. Commun., 6,
67-75.
29.Morris, J. C. (1966) J. Phys. Chem.,
70, 3798-3805.
30.Arnhold, J., Panasenko, O. M., Schiller, J.,
Vladimirov, Yu. A., and Sergienko, V. I. (1995) Chem. Phys.
Lipids, 78, 55-64.
31.Govorova, N. Yu., Sharonov, B. P., and Lyzlova,
S. N. (1988) Biokhimiya, 53, 2025-2032.
32.Arnhold, J., Wiegel, D., Richter, O.,
Hammerschmidt, S., Arnold, K., and Krumbiegel, M. (1991) Biomed.
Biochim. Acta, 50, 967-973.
33.van den Berg, J. J. M., Winterbourn, C. C., and
Kuypers, F. A. (1993) J. Lipid Res., 34, 2005-2012.
34.Heinecke, J. W., Li, W., Mueller, D. M., Boherer,
A., and Turk, J. (1994) Biochemistry, 33,
10127-10136.
35.Panasenko, O. M., Evgina, S. A., and Sergienko,
V. I. (1993) Byull. Eksp. Biol. Med., 116, 153-155.
36.Panasenko, O. M., Evgina, S. A., Nikitin, S. V.,
and Sergienko, V. I. (1993) Biofizika, 40, 545-550.
37.Esterbauer, H., Gebicki, J., Puhl, H., and
Jürgens, G. (1992) Free Radical Biol. Med., 13,
341-390.
38.Motchnik, P. A., Frei, B., and Ames, B. N.
(1994) Meth. Enzymol., 234, 269-279.
39.Fliss, H., and Menard, M. (1991) Arch.
Biochem. Biophys., 287, 175-179.
40.Shaish, A., Daugherty, A., O'Sullivan, F.,
Schonfeld, G., and Heinecke, J. W. (1995) J. Clin. Invest.,
96, 2075-2082.