Comparative Study on Monoclonal Immunoglobulin M and Rheumatoid Immunoglobulin M by Differential Scanning Microcalorimetry
I. I. Protasevich,1 B. Ranjbar,1 E. Yu. Varlamova,2 I. A. Cherkasov,3 and V. A. Lapuk3,4
1Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, ul. Vavilova 32, Moscow, 117984 Russia; fax: (095) 135-1405.2Hematology Research Center, Russian Academy of Medical Sciences, Novozykovskii Proezd 4a, Moscow, 125167 Russia; fax: (095) 212-4252.
3Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninskii pr. 47, Moscow, 117913 Russia; fax: (095) 135-5328.
4To whom correspondence should be addressed.
Submitted March 19, 1997.
Thermal denaturation of monoclonal immunoglobulin M (IgM) and rheumatoid immunoglobulin M (IgM-RF) and their Fab- and (Fc)5-fragments was studied by differential scanning microcalorimetry. The melting of IgM-RF started at a higher temperature than that of IgM and the maximum temperature of its main asymmetric peak of heat absorption was higher by 4°C. At equal values of enthalpy, the thermal denaturation of IgM-RF and IgM consisted of four and five individual transitions, respectively, between pairs of states. The comparison of thermal denaturation parameters of Fab- and (Fc)5-fragments of IgM-RF and IgM showed a thermodynamic similarity of (Fc)5-fragments of both proteins, while their Fab-fragments differed in the interaction between VL-CL and VH-CH domains.
KEY WORDS: rheumatoid immunoglobulin M, immunoglobulin M, Fab- and (Fc)5-fragments, thermal denaturation, scanning microcalorimetry.
Abbreviations: IgM) monoclonal immunoglobulin M; IgM-RF) IgM possessing rheumatoid activity; DSM) differential scanning microcalorimetry; Td) denaturation temperature; deltaHd) denaturation enthalpy; Tt) temperature of transition between two states; deltaHt) enthalpy of transition between two states; deltahd) denaturation specific heat.
A specific feature of IgM-RF5 is its ability to form an
immune complex with IgG in vivo [1].
Nevertheless, there are very few reliable data on differences in the
structure of IgM-RF and usual IgM not possessing the rheumatoid
activity. Thus, it seems important to detect interrelations between the
structure and pathological function of IgM-RF, in particular, for
development of approaches to diagnosis and treatment of rheumatoid
arthritis and other autoimmune diseases. Based on results of
sedimentation analysis, we suggested that the Fab-fragments of IgM-RF
are nonequivalent in function and structure [2]. To
continue these studies, we used the DSM method to compare thermal
denaturation of IgM (Waldenstrom's disease), IgM-RF, and their Fab- and
(Fc)5-fragments. This methods provides information about the
true thermodynamic parameters of conformational transitions in
proteins. The DSM method, in particular, detects intermediate states
during the thermal denaturation of molecules of multidomain structure
and determines the level of interaction between separate structural
domains in an intact molecule [3, 4]. It is of special importance for immunoglobulins of
such structure because the antigen binding and effector functions of
immunoglobulins are regulated by a number of conformational transitions
which affect interdomain contacts [1, 5]. Tischenko et al. using the DSM method to study
rabbit IgG suggested a model of interaction between its structural
domains which form cooperatively melting units [6].
IgM and IgM-RF consist of five subunits homologous to an IgG molecule
[7] and, consequently, are significantly more
elaborated systems than IgG from the viewpoint of scrupulous
interpretation of their unfolding/refolding mechanism. The purpose of
this work was to comparatively analyze the microcalorimetric melting of
intact molecules of IgM and IgM-RF and of their fragments. The thermal
denaturations of IgM and IgM-RF were found to differ significantly, and
their Fab-fragments were the most distinct in their denaturation
parameters; thus, these fragments are suggested to differ in
interdomain interactions.
MATERIALS AND METHODS
Human IgM was isolated as described earlier [8]. Human IgM-RF was obtained by our method developed earlier [9]. The proteins were purified by gel filtration on Sepharose CL-4B and in the further work only fractions corresponding to the region of maximum and consequent abating of the elution peak were used because only these fractions contained no associates as judged from ultracentrifugation data [2].
The preparations of IgM and IgM-RF were fragmented with "hot trypsin" (58-60°C) (Spofa, Czechoslovakia) and the appropriate Fab- and (Fc)5-fragments were isolated by the method described in [8] with Sephacryl S-300 instead of Biogel A-1.5 m.
The protein solutions were concentrated in an ultrafiltration cell of a model 8050 (Amicon, Holland) with a Diaflo PM-30 membrane of the same firm. Immediately before the calorimetry, the solution samples were filtered through a ME 25 membrane filter (the pore diameter 0.45 µm) (Schleicher and Schuell, Germany). The protein concentration was determined spectrophotometrically at 280 nm using the value of A1%1cm,280 = 12 [10].
Tris (Merck, Germany); Sephacryl S-300 and Sepharose CL-4B (Pharmacia, Sweden) were used; other reagents were of domestic production and were of chemical or analytical purity.
All calorimetric experiments were carried out in 0.01 M potassium phosphate buffer (pH 7.0) containing 0.15 M NaCl. Before the measurements, the protein solutions were dialyzed against this buffer for 24 h at 4°C. The measurements were performed with a DASM-4 differential scanning microcalorimeter (Biopribor, Russia) in cells of 0.48 ml at the rate of 1°K/min of solution heating. To prevent the degassing of solutions during the heating, an excess pressure of 1.5 atm was maintained. The protein concentration was 0.8-1.8 mg/ml. Thermal denaturation of immunoglobulins and their fragments did not depend on their concentration. The heating curves of the protein solutions were corrected considering the base line of the device which was obtained by the heating of the solvent which was used for preparation and dialysis of the protein solutions. Reversibility of the denaturation curves was determined by a second heating of the same sample after cooling in the calorimetric cells. Calorimetric denaturation enthalpy, deltaHd, was determined according to [11] assuming the following: molecular mass of each IgM/IgM-RF subunit, 184 kD; those of Fab-fragments of IgM and IgM-RF, 49 kD; and of Fc-fragments of IgM and IgM-RF, 70.3 and 90.6 kD, respectively [2, 12-14]. The temperature function of excess heat capacity was analyzed with a SCAL2 program (Institute of Protein Research, Russian Academy of Sciences, Pushchino) [15, 16] which is a modification of the method described in [17]. This program permits determination of the number of independent transitions between two states (further denoted as transitions) which produces the complex melting endotherm without preliminary assumptions concerning the mechanism of the unfolding of the macromolecule. The error of determination of the denaturation temperature, Td, was ±0.2°C, of the denaturation enthalpy, deltaHd, ±6%. The determination errors for parameters of individual transitions obtained by deconvolution of the complex melting curves were not more than ±8% for the transition enthalpy (deltaHt) and ± 3°C for the transition temperature (Tt).
RESULTS AND DISCUSSION
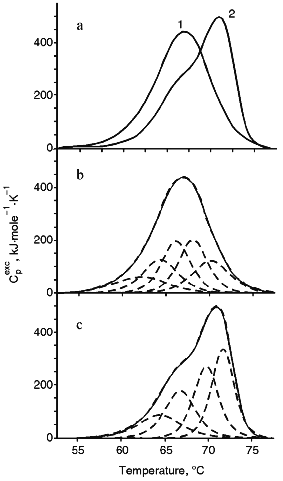
Thermal Denaturation of Intact IgM and IgM-RF. The melting of IgM at pH 7.0 displays a wide peak of heat absorption in the temperature interval of 52-75°C with a maximum at 67.1°C (Fig. 1a, curve 1). The melting of IgM-RF (Fig. 1a, curve 2) started at a higher temperature; its maximum temperature was 4°C higher than that of IgM, and its peak of heat absorption was of more pronounced asymmetry. All calorimetrical curves were well reproducible, but the thermal denaturation of proteins was irreversible. Since the calorimetry failed to detect an appreciable aggregation and the thermal denaturation parameters did not depend on the protein concentration, the events after denaturation which were possibly responsible for the irreversibility of denaturation were suggested to occur more slowly than the unfolding of the molecule. Results of quantitative analysis of melting curves of the studied immunoglobulins are presented in Table 1. Since molecules of immunoglobulin M consist of five identical subunits [7], the melting enthalpy, deltaHd, was calculated based on the subunit molecular mass of 184 kD for both IgM and IgM-RF. Table 1 shows that values of deltaHd were equal for IgM and IgM-RF. Thus, the increased thermal stability of IgM-RF versus IgM seems to be caused by changes in the entropy. Based on the curves of temperature dependence of the excess heat capacity, i.e., the width of the temperature interval and the peak asymmetry (Fig. 1a, curves 1 and 2), intermediate thermodynamically stable states were suggested to exist during the unfolding of each subunit of IgM and IgM-RF [11]. Based on this, the resulting calorimetric curves were deconvoluted. Five successive transitions were obtained for IgM (Fig. 1b) and four transitions for IgM-RF (Fig. 1c). Attempts to describe the experimental calorimetric curves of the excess heat capacity using the corresponding theoretical curves which are sums of a number of transitions resulted in significantly increased deviation of the theoretical and experimental values. Since the structure of IgM and IgM-RF subunits is similar to that of IgG [7], the data of this work were compared to results of calorimetric studies on IgG. Complex calorimetric melting curves of rabbit IgG at pH 2.5-3.5 [6] and of mouse IgG1 (MAK33) at pH 2.0-7.0 [18] were earlier obtained and divided into separate transitions. The value of deltaHd for MAK33 at pH 7.0 was close to our data for IgM and IgM-RF subunits (Table 1). It should be noted that the number of deconvolution peaks of the melting curve of IgM (Fig. 1b) was equal to the number of peaks obtained for immunoglobulin MAK33 at pH 7.0. Unlike the curve of IgM, the melting curve of IGM-RF showed four transitions (Fig. 1c). Results of deconvolution are presented in Table 2. To compare the calorimetric behavior of both immunoglobulins of complex structure and their separate parts able to interact, the data of Table 2 were analyzed based on the suggestion that denaturation of a protein passes through a number of intermediate thermodynamically stable states (four and three intermediate states for IgM and IgM-RF subunits, respectively). Thus, unlike the work published in [6], the melting of some domains during thermal denaturation of intact IgM and IgM-RF molecules was not postulated. The results presented in Tables 1 and 2 show that the absence of one deconvolution peak in the case of IgM-RF as compared to IgM failed to decrease the total enthalpy of denaturation. It seems to indicate that the unfolding of the denatured molecules of both immunoglobulins are virtually the same. The temperatures of the first, second, and fourth transitions of the IgM-RF subunit were found to be close to temperatures of the second, third, and fifth transitions of the IgM subunit. The difference between temperatures of the third and fourth transitions of IgM-RF and IgM, respectively, was greater than the determination error of this parameter during the deconvolution. Enthalpies of the first and third transitions of IgM-RF were different from enthalpies of the second and fourth transitions of IgM by -17 and +16%, respectively, while the enthalpy of the second transition of IgM-RF was virtually the same as the enthalpy of the third transition of IgM. The greatest difference between the transition enthalpies of IgM-RF and IgM at very close temperature values was found for transitions from the last intermediate into the denatured state of both immunoglobulins. The value of this difference was consistent with that of deltaHt of the transition between the native and first intermediate states at Tt = 63.2°C when no transition took place in IgM-RF. Thus, the thermal denaturation of an intact molecule of IgM-RF differed from that of IgM mainly by the absence of one intermediate thermodynamically stable state which was described by the lowest Tt and the significantly greater value of deltaHt (by more than 70%) of the transition between the last intermediate and the denatured states, i.e., at the maximal Tt. To elucidate the cause of this difference, we studied the thermal denaturation of Fab- and Fc-fragments as simpler systems than subunits of intact IgM and IgM-RF.
TABLE 1. Parameters of Thermal Denaturation of IgM, IgM-RF, and Their Fab- and Fc-FragmentsFig. 1. a) Temperature function of the excess heat capacity of IgM (1) and IgM-RF (2); b) results of deconvolution of the temperature function of the excess heat capacity of IgM; c) the same for IgM-RF. The experimental curve is shown with the solid line, the deconvoluted peaks and their sum are shown with the dashed lines.

TABLE 2. Thermodynamic Parameters of
Transitions Obtained by Deconvolution of Temperature Function of Excess
Heat Capacity of IgM, IgM-RF, and Their Fragments
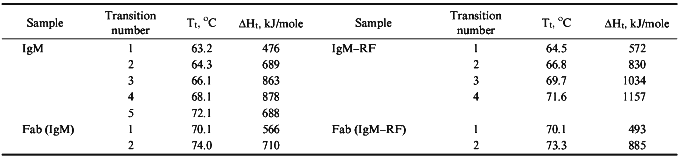
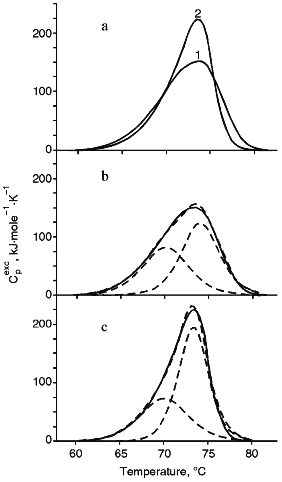
Melting of Fab-Fragments of IgM and IgM-RF. The melting endotherms of Fab-fragments of both immunoglobulins were asymmetric, but the shapes of the curves differed significantly (Fig. 2a), and values of Td and deltaHd were very similar (Table 1). Isolated Fab-fragments of IgM and IgM-RF melted at temperatures 6.6 and 2.2°C higher, respectively, than those of the intact immunoglobulin molecules, and the denaturation heat of Fab-fragments per unit of protein mass (deltahd) was, on average, 40% greater than the corresponding value for the intact subunits. Therefore, this suggests that either the denatured Fab-fragments are unfolded to a greater extent than the denatured immunoglobulin molecule, or the unfolding of the latter was accompanied by a greater negative contribution into the denaturation enthalpy due to destruction of hydrophobic interactions. Results of deconvolution analysis of the temperature function of the excess heat capacity of Fab-fragments are shown in Fig. 2, b and c. The melting of Fab-fragments of both IgM and IgM-RF are clearly represented by two transition peaks. The temperature values of the corresponding transitions of the two immunoglobulins were similar. However, the values of transition enthalpy differed: the value of deltaHt for the low temperature peak of the Fab-fragment of IgM-RF was 15% lower than that of the Fab-fragment of IgM, while for the high temperature peak this value was higher by 25% (Table 2). Taking into account the high structural homology of Fab-fragments of IgM and IgG, we compared our results with the data of [6]. In that research the total melting enthalpy of the Fab-fragment of IgG was virtually unchanged and was 1300 kJ/mole, while the transition temperature changed by 6-7°C on changing the solution pH from 2.5 to 3.5. As shown in Table 1, the deltaHd for melting of the Fab-fragments of IgM and of IgG at pH 7.0 were the same, but the calorimetrical curve of the Fab-fragment of IgM had one less division peak. Tischenko et al. [6] concluded that the first of three melting peaks (the low temperature peak) of the Fab-fragment of IgG was associated with the destruction of contacts between VL-CL and VH-CH domains, while the other two peaks represented the melting of cooperative blocks from the pair of variable domains (VL, VH) and the pair of constant domains (CL, CH1). The destruction of contacts responsible for the interaction between the VL-CL and VH-CH domains in the Fab-fragment of immunoglobulins M seems to be a non-cooperative process, and its enthalpy is distributed between enthalpies of two melting peaks of cooperative blocks. Since the deltaHt at the melting of the Fab-fragment of IgM-RF is 15% lower for the low temperature peak and 25% higher for the high temperature peak than for the Fab-fragment of IgM (Table 2), the enthalpy of interaction between the VL-CL and VH-CH domains appears to be mainly included into the enthalpy of the high temperature transition. Thus, the thermal denaturation of the Fab-fragment of IgM-RF differed from that of the Fab-fragment of IgM by the difference in enthalpy values of cooperative transitions which described the different interaction between the VL-CL and VH-CH domains.
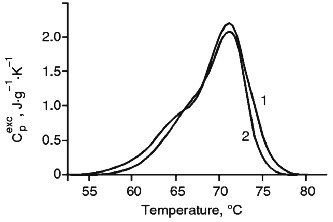
Melting of (Fc)5-Fragments of IgM and IgM-RF. Results of melting of (Fc)5-fragments of IgM and IgM-RF are presented in Fig. 3. The melting endotherms for both cases are asymmetric curves of similar shape with insignificantly different amplitude at the maximum temperature. According to results of [2], the (Fc)5-fragment of IgM-RF should contain two undetached Fab-fragments. Therefore, we did not deconvolute the temperature function of the excess heat capacity of the (Fc)5-fragment of IgM. Table 1 shows that the (Fc)5-fragment of IgM melted at a temperature 4.1°C higher than Td of an intact IgM molecule and the (Fc)5-fragment of IgM-RF melted at a temperature close to Td of the intact IgM-RF molecule. But the melting temperature of the (Fc)5-fragment of both immunoglobulins was lower than that of their Fab-fragments. This result is consistent with data for rabbit IgG [6]. The melting enthalpy of an intact IgM molecule calculated by summing over its values for Fab- and (Fc)5-fragments was in good agreement with results of measurements for intact molecules. Parameters of heat denaturation (Td, deltahd) of Fc-fragments of IgM and IgM-RF were very close (Table 1); therefore, the (Fc)5-fragment of IgM, from the viewpoint of molecular energetics, should be considered as a conservative part of molecule which does not significantly differ from that of a molecule of IgM-RF.Fig. 2. a) Temperature function of the excess heat capacity of Fab-fragments: 1) of IgM; 2) of IgM-RF; b) results of deconvolution of the temperature function of the excess heat capacity for Fab-fragments of IgM; c) the same for Fab-fragments of IgM-RF. The experimental curve is shown with the solid line, the deconvoluted peaks and their sum are presented with the dashed lines.
Thus, an intact IgM-RF molecule was more thermostable than an IgM molecule and was distinguished by the absence of one of four intermediate thermodynamically stable states and by the significantly higher value of enthalpy of the transition between the last intermediate and the denatured states. The comparison of the heat denaturation parameters of Fab- and (Fc)5-fragments of IgM-RF and IgM suggest that the (Fc)5-fragments of both proteins are thermodynamically similar and their Fab-fragments differ by the interaction between the VL-CL and VH-CH domains.Fig. 3. Temperature function of the excess heat capacity of (Fc)5-fragments: 1) IgM; 2) IgM-RF.
The work was supported by the Russian Foundation for Basic Research (grants No. 966-04-49526 and No. 96-04-50330).
LITERATURE CITED
1.Kehoe, J. M. (1978) in Comprehensive
Immunology, Vol. 5, Immunoglobulins (Litman, G. W., and Good, R.
A., eds.) Plenum Medical Book Co., New York-London, pp. 173-196.
2.Lapuk, V. A., Chernyak, V. Ya., and Magretova, N.
N. (1996) Biochemistry (Moscow), 61, 85-88 (Russ.).
3.Makarov, A. A., Protasevich, I. I., Frank, E. G.,
Grishina, I. B., Bolotina, I. A., and Esipova, N. G. (1991) Biochim.
Biophys. Acta, 1078, 283-288.
4.Protasevich, I. I., Ranjbar, B., Lobachov, V. M.,
Makarov, A. A., Gilli, R., Briand, C., Lafitte, D., and Haiech, J.
(1997) Biochemistry, 36, 11401-11408.
5.Creighton, T. E. (1990) Biochem. J.,
270, 1-16.
6.Tischenko, V. M., Zav'yalov, V. P., Medgyesi, G.
A., Potekhin, S. A., and Privalov, P. L. (1982) Eur. J.
Biochem., 126, 517-521.
7.Turner, M. (1983) in Immunochemistry. An
Advanced Textbook (Glynn, L. E., and Steward, M. V., eds.) John
Wiley and Sons, Chichester-New York-Brisbane-Toronto, pp. 1-57.
8.Lapuk, V. A., Khatiashvili, M. M., Chukhrova, A.
I., and Kaverzneva, E. D. (1985) Biokhimiya, 50,
237-242.
9.Lapuk, V. A., Chukhrova, A. I., Chernokhvostova, E.
V., Aleshkin, V. A., German, G. P., Varlamova, E. Yu., Ponomareva, A.
M., Arbatskii, N. P., and Zheltova, A. O. (1992) Biokhimiya,
57, 617-626.
10.Ghose, A. C. (1971) Biochem. Biophys. Res.
Commun., 45, 1144-1150.
11.Privalov, P. L., and Potekhin, S. A. (1986)
Meth. Enzymol., 131, 4-51.
12.Putman, F. W., Florent, G., Paul, C., Shinoda,
T., and Shimizu, A. (1973) Science, 182, 287-291.
13.Laure, C. J., Watanabe, S., and Hilschmann, N.
(1973) Hoppe-Seyler's Z. Physiol. Chem., 354,
1503-1504.
14.Florent, G., Lehman, D., Lockhart, D., and
Putman, F. W. (1974) Biochemistry, 13, 3372-3381.
15.Filimonov, V. V., Potekhin, S. A., Matveev, S.
V., and Privalov, P. L. (1982) Mol. Biol. (Moscow), 16,
435-444.
16.Filimonov, V. V., Prieto, J., Martinez, J. C.,
Bruix, M., Mateo, P. L., and Serrano, L. (1993) Biochemistry,
32, 12906-12921.
17.Freire, E., and Biltonen, R. L. (1978)
Biopolymers, 17, 463-479.